Abstract
Background
Infertility is a condition affecting 10% to 15% of couples of reproductive age. It is generally defined as "the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse". The treatment of infertility may involve manipulation of gametes or of the embryos themselves. These techniques are together known as assisted reproductive technology (ART). Practitioners are constantly seeking alternative or adjunct treatments, or both, in the hope that they may improve the outcome of assisted reproductive techniques. This Cochrane review focusses on the adjunct use of synthetic versions of two naturally‐produced hormones, dehydroepiandrosterone (DHEA) and testosterone (T), in assisted reproduction.
DHEA and its derivative testosterone are steroid hormones proposed to increase conception rates by positively affecting follicular response to gonadotrophin stimulation, leading to greater oocyte yields and, in turn, increased chance of pregnancy.
Objectives
To assess the effectiveness and safety of DHEA and testosterone as pre‐ or co‐treatments in subfertile women undergoing assisted reproduction.
Search methods
We searched the following electronic databases, trial registers and websites up to 12 March 2015: the Cochrane Central Register of Controlled Trials (CENTRAL), the Menstrual Disorders and Subfertility Group (MDSG) Specialised Register, MEDLINE, EMBASE, PsycINFO, CINAHL, electronic trial registers for ongoing and registered trials, citation indexes, conference abstracts in the Web of Science, PubMed and OpenSIGLE. We also carried out handsearches. There were no language restrictions.
Selection criteria
We included randomised controlled trials (RCTs) comparing DHEA or testosterone as an adjunct treatment to any other active intervention, placebo, or no treatment in women undergoing assisted reproduction.
Data collection and analysis
Two review authors independently selected studies, extracted relevant data and assessed them for risk of bias. We pooled studies using fixed‐effect models. We calculated odds ratios (ORs) for each dichotomous outcome. Analyses were stratified by type of treatment. There were no data for the intended groupings by dose, mode of delivery or after one/more than one cycle.
We assessed the overall quality of the evidence for the main findings using the GRADE working group methods.
Main results
We included 17 RCTs with a total of 1496 participants. Apart from two trials, the trial participants were women identified as 'poor responders' to standard IVF protocols. The included trials compared either testosterone or DHEA treatment with placebo or no treatment.
When DHEA was compared with placebo or no treatment, pre‐treatment with DHEA was associated with higher rates of live birth or ongoing pregnancy (OR 1.88, 95% CI 1.30 to 2.71; eight RCTs, N = 878, I² statistic = 27%, moderate quality evidence). This suggests that in women with a 12% chance of live birth/ongoing pregnancy with placebo or no treatment, the live birth/ongoing pregnancy rate in women using DHEA will be between 15% and 26%. However, in a sensitivity analysis removing trials at high risk of performance bias, the effect size was reduced and no longer reached significance (OR 1.50, 95% CI 0.88 to 2.56; five RCTs, N = 306, I² statistic = 43%). There was no evidence of a difference in miscarriage rates (OR 0.58, 95% CI 0.29 to 1.17; eight RCTs, N = 950, I² statistic = 0%, moderate quality evidence). Multiple pregnancy data were available for five trials, with one multiple pregnancy in the DHEA group of one trial (OR 3.23, 95% CI 0.13 to 81.01; five RCTs, N = 267, very low quality evidence).
When testosterone was compared with placebo or no treatment we found that pre‐treatment with testosterone was associated with higher live birth rates (OR 2.60, 95% CI 1.30 to 5.20; four RCTs, N = 345, I² statistic = 0%, moderate evidence). This suggests that in women with an 8% chance of live birth with placebo or no treatment, the live birth rate in women using testosterone will be between 10% and 32%. On removal of studies at high risk of performance bias in a sensitivity analysis, the remaining study showed no evidence of a difference between the groups (OR 2.00, 95% CI 0.17 to 23.49; one RCT, N = 53). There was no evidence of a difference in miscarriage rates (OR 2.04, 95% CI 0.58 to 7.13; four RCTs, N = 345, I² = 0%, low quality evidence). Multiple pregnancy data were available for three trials, with four events in the testosterone group and one in the placebo/no treatment group (OR 3.09, 95% CI 0.48 to 19.98; three RCTs, N = 292, very low quality evidence).
One study compared testosterone with estradiol and reported no evidence of a difference in live birth rates (OR 2.06, 95% CI 0.43 to 9.87; one RCT, N = 46, very low quality evidence) or miscarriage rates (OR 0.70, 95% CI 0.11 to 4.64; one RCT, N = 46, very low quality evidence).
The quality of the evidence was moderate, the main limitations being lack of blinding in the included trials, inadequate reporting of study methods, and low event and sample sizes in some trials.
Authors' conclusions
In women identified as poor responders undergoing ART, pre‐treatment with DHEA or testosterone may be associated with improved live birth rates. The overall quality of the evidence is moderate. There is insufficient evidence to draw any conclusions about the safety of either androgen. Definitive conclusions regarding the clinical role of either androgen awaits evidence from further well‐designed studies.
Plain language summary
Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction
Review question
Researchers in the Cochrane collaboration reviewed the evidence about the effectiveness and safety of androgens in women undergoing assisted reproduction. The androgens evaluated were dehydroepiandrosterone (DHEA) and testosterone (T) as pre‐ or co‐treatments aiming to improve pregnancy and live birth rates in those women.
Background
Infertility affects 10% to 15% of couples seeking to start a family. DHEA and T are steroid hormones that researchers have suggested might increase conception rates by increasing the response of the ovary to stimulation.
Study characteristics
This Cochrane review included 17 randomised controlled trials which compared treatment with the androgens DHEA or T with placebo or no treatment in a total of 1496 women, almost all of whom had been identified as 'poor responders' to standard assisted reproduction protocols. The main outcomes were live birth (defined as delivery of a live baby after 20 weeks gestation) or ongoing pregnancy rates, miscarriage, clinical pregnancy rates (fetal heartbeat confirmed on ultrasound) and multiple pregnancy rates. We examined the evidence published up to 12 March 2015.
Key results
DHEA and T use may be associated with increased live birth rates. The evidence for the use of DHEA suggested that in women with a 12% chance of live birth with placebo or no treatment, the live birth rate in women using DHEA will be between 15% and 26%. The evidence for the use of T suggested that in women with an 8% chance of live birth with placebo or no treatment, the live birth rate in women using T will be between 10% and 32%. When we removed from the analyses the studies at high risk of bias, this increase was no longer present for DHEA or T. There is insufficient evidence to draw any conclusions about the safety of either androgen.
Quality of the evidence
The quality of the trials was moderate, and the main limitations were lack of blinding, inadequate reporting of study methods and small sample sizes in some included trials.
Summary of findings
Summary of findings for the main comparison. DHEA or testosterone versus placebo/no treatment for women undergoing assisted reproduction.
| DHEA or testosterone versus placebo/no treatment for women undergoing assisted reproduction | ||||||
| Population: Women undergoing assisted reproduction Settings: Outpatient clinic Intervention: DHEA or testosterone versus placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | DHEA or testosterone | |||||
| Live birth/ongoing pregnancy rate ‐ DHEA | 116 per 1000 | 192 per 1000 (141 to 256) | OR 1.81 (1.25 to 2.62) | 878 (8 studies) | ⊕⊕⊕⊝ moderate1 | |
| Live birth/ongoing pregnancy rate ‐ Testosterone | 82 per 1000 | 188 per 1000 (104 to 317) | OR 2.6 (1.3 to 5.2) | 345 (4 studies) | ⊕⊕⊕⊝ moderate1 | |
| Miscarriage rate ‐ DHEA | 64 per 1000 | 38 per 1000 (19 to 74) | OR 0.58 (0.29 to 1.17) | 950 (8 studies) | ⊕⊕⊕⊝ moderate1 | |
| Miscarriage rate ‐ Testosterone | 25 per 1000 | 50 per 1000 (15 to 155) | OR 2.04 (0.58 to 7.13) | 345 (4 studies) | ⊕⊕⊝⊝ low1,2 | |
| Clinical pregnancy rate ‐ DHEA | 208 per 1000 | 260 per 1000 (210 to 316) | OR 1.34 (1.01 to 1.76) | 1246 (12 studies) | ⊕⊕⊕⊝ moderate1 | |
| Clinical pregnancy rate ‐ Testosterone | 115 per 1000 | 247 per 1000 (150 to 378) | OR 2.52 (1.36 to 4.68) | 345 (4 studies) | ⊕⊕⊕⊝ moderate1,3 | |
| Multiple pregnancy ‐ DHEA | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.23 (0.13 to 81.01) | 267 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Multiple pregnancy ‐ Testosterone | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.09 (0.48 to 19.98) | 292 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| *The basis for the assumed risk is the median risk in the control groups. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Some small sample sizes and low total numbers of events. 2Very wide CIs. 3Downgraded two levels for imprecision.
Summary of findings 2. Testosterone compared to other active interventions for women undergoing assisted reproduction.
| Testosterone compared to other active interventions for women undergoing assisted reproduction | ||||||
| Population: Women undergoing assisted reproduction Settings: Outpatient clinic Intervention: Testosterone Comparison: Estradiol, with or without OCP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other active interventions | Testosterone | |||||
| Live birth ‐ Testosterone vs estradiol | 125 per 1000 | 227 per 1000 (58 to 585) | OR 2.06 (0.43 to 9.87) | 46 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
| Live birth ‐ Testosterone vs estradiol + OCP | 150 per 1000 | 228 per 1000 (57 to 588) | OR 1.67 (0.34 to 8.10) | 42 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
| Miscarriage ‐ Testosterone vs estradiol | 125 per 1000 | 91 per 1000 (15 to 399) | OR 0.70 (0.11 to 4.64) | 46 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
| Miscarriage ‐ Testosterone vs estradiol + OCP | 50 per 1000 | 91 per 1000 (8 to 545) | OR 1.90 (0.16 to 22.72) | 42 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
| Clinical pregnancy rate ‐ Testosterone vs estradiol | 250 per 1000 | 318 per 1000 (115 to 628) | OR 1.40 (0.39 to 5.07) | 46 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
| Clinical pregnancy rate ‐ Testosterone vs estradiol + OCP | 200 per 1000 | 319 per 1000 (101 to 658) | OR 1.87 (0.45 to 7.69) | 42 (1 RCT) | ⊕⊝⊝⊝ very low1,2 | |
| *The basis for the assumed risk is based on the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Methods of allocation concealment not clearly described. 2Single small study with few events, CIs compatible with substantial benefit or harm from the intervention, or with no effect.
Background
Description of the condition
Subfertility is a condition affecting 10% to 15% of couples of reproductive age (Gnoth 2005). It is generally defined as "the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse" (Zegers‐Hochschild 2009).
The treatment of subfertility may be specific to the causative medical or surgical disorder or, instead, may involve manipulation of gametes/embryos themselves. The latter technique is known as assisted reproductive technology (ART), which is defined as "all treatments or procedures that include the in vitro handling of both human oocytes and sperm or of embryos for the purpose of establishing a pregnancy" (Zegers‐Hochschild 2009); in vitro fertilisation (IVF) is one of them.
Despite substantial improvements in the success of these treatments since the inception of IVF (Toner 2002), live birth rates vary from 20% to 50%, and practitioners are constantly seeking for adjunct treatments to improve the outcomes, either in the form of medical (Akhtar 2013; Siristatidis 2011) or non‐medical (Cheong 2013) co‐therapies. This Cochrane review focuses on the adjunct use of synthetic versions of two naturally‐produced hormones, dehydroepiandrosterone (DHEA) and testosterone (T), in assisted reproduction.
Description of the intervention
DHEA and its derivative, testosterone, are steroid hormones proposed to increase the conception rates in women undergoing ART (Barad 2007).
DHEA is an androgen pre‐hormone produced in the zona reticularis of the female adrenal gland and the ovarian theca cells. It is a precursor to the sex hormones testosterone and estradiol. It is first produced during fetal life, while serum levels of DHEA decrease markedly after the 45th year of women's age (Buvat 2003; Davison 2005). Its anti‐aging effects were described 30 years ago (Morales 1994), which led to further investigation into the role of DHEA in improving ovarian reserve. It was first reported as a treatment in ART in 2000, being used as an adjunct to IVF in women with premature ovarian failure (POF), premature ovarian aging (POA) and diminished ovarian reserve (DOR) (Casson 2000). It has been demonstrated to improve IVF outcomes in women with poor ovarian function and increased follicle stimulating hormone (FSH) levels, when administered prior to and during an IVF cycle (Barad 2006). DHEA, used as an oral preparation in a variety of doses, appears to increase the number of oocytes produced leading to improvements in pregnancy rates, both in intrauterine insemination and IVF cycles (Barad 2007; Gleicher 2011a), while reducing miscarriage rates in women with diminished ovarian reserve undergoing IVF (Gleicher 2009). Despite its widespread use as an adjuvant in ART, there remains uncertainty about the true efficacy of DHEA.
The sex hormone T has also been used as an adjunct in ART (Balasch 2006; Kim 2010). It is administered directly, either trans dermally (skin patch or gel), orally, or as a subcutaneous implant. Its use has been reported in poor responders, but results are conflicting. The optimal dose or duration of T administration, or both, has not as yet been established.
Notably, DHEA and T may be associated with androgenic side‐effects in female users. Exogenous administration of T may influence sexual desire, bone mineral density, muscle mass, adipose tissue distribution, mood, energy and psychological well‐being (Somboonporn 2005). Importantly, the effect of their periconceptual use on the developing embryo is of concern (Sir‐Petermann 2002). A post‐traumatic seizure was reported in a woman taking DHEA with a history of previous brain injury (Karp 2009). Available data suggest that a patient's medical history along with the administration method and dosage of the androgens require further investigation.
How the intervention might work
The potential mechanisms by which DHEA and T could increase pregnancy rates in ART are elusive and still under investigation. Androgens play a crucial role in maintaining adequate follicular steroidogenesis by acting as a substrate for the conversion of androgens to estrogens through aromatase (Ryan 1968).
Also, they exert an inert role in pre‐antral and small antral follicular physiology and function, increasing their numbers (Weil 1998; Weil 1999), or by direct autocrine and paracrine effects on regulation of follicular function (Horie 1992). Notably, they promote the induction and up‐regulation of FSH and androgen receptors in pre‐antral and antral follicles, thus preventing granulosa cell atresia and amplifying the effect of FSH on follicular growth (Garcia‐Velasco 2005; Nielsen 2011; Vendola 1999; Weil 1998; Sen 2010).
A potential mechanism of action of both androgens is by increasing the follicular insulin‐like growth factor‐I (IGF‐I) (Genazzani 2001; Mamas 2009a): administration of T in animals causes an increase in IGF‐I levels and a decrease in insulin‐like growth factor‐binding protein 1 (IGFBP‐1) levels (Vendola 1999; Yakin 2011). Similarly, in postmenopausal women, IGF‐I levels were found to have increased after three months of use, albeit there was a drop at six months (Casson 1998). IGF‐1 enhances gonadotrophin action, leading to greater oocyte yields, and consequently to improved pregnancy outcomes (Gleicher 2011b; Mamas 2009b).
Another postulated mechanism for these androgens is as pre‐hormones for follicular fluid testosterone, as they act as a ligand for androgen receptors, promoting ovarian follicular growth. Practically it has been suggested that their administration creates a "polycystic ovary syndrome (PCOS) like" environment that leads to an increase in small antral follicles and anti‐Müllerian hormone (AMH) levels (Fouany 2013). Notably, reduced levels of endogenous androgens have been associated with decreased ovarian sensitivity to FSH and low pregnancy rates in IVF cycles (Frattarelli 2004).
The presumption is that increasing androgen levels by the exogenous administration of DHEA or T in subfertile women undergoing ART might lead to an improvement in ART outcomes.
Why it is important to do this review
The use of DHEA and testosterone in ART is increasing as they may improve the chances of conception in subfertile women. These products are inexpensive and simple to use, yet remain controversial because of a lack of robust evidence for their efficacy and safety. In this Cochrane review we will summarise the available evidence on the use of DHEA and testosterone in subfertile women who are undergoing ART and identify any gaps or limitations in our current understanding.
Objectives
To assess the effectiveness and safety of DHEA and testosterone as pre‐ or co‐treatments in subfertile women undergoing assisted reproduction.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs). Studies with evidence of inadequate sequence generation, such as allocation by date of birth or hospital number, were excluded. We included crossover trials for completeness, but we included data from the first phase only in any meta‐analyses as the crossover design was not valid in this context.
Types of participants
We included women undergoing IVF or intra‐cytoplasmic sperm injection (ICSI).
Exclusion criteria included:
Women who were peri‐ or post‐menopausal;
Women already taking DHEA or T at time of enrolment.
Types of interventions
Trials using DHEA or testosterone as an adjunct treatment versus any other active intervention, placebo or no treatment were eligible for inclusion.
Types of outcome measures
Primary outcomes
Live birth or ongoing pregnancy rate per woman randomised: we defined live birth as delivery of a live fetus after 20 completed weeks of gestation; we defined ongoing pregnancy as a pregnancy beyond 20 weeks of gestation.
Miscarriage rate per woman randomised, defined as the number of pregnancies lost before 20 weeks of gestation.
Secondary outcomes
Clinical pregnancy rate per woman randomised, defined as evidence of a gestational sac on ultrasound.
Adverse effects to the woman (per woman randomised: ectopic pregnancy, multiple birth, antenatal and perinatal complications; this includes adverse events resulting directly from the treatment administered).
Adverse fetal effects including fetal anomalies (chromosomal, congenital and anatomical abnormalities, preterm labour, growth restriction).
Search methods for identification of studies
We developed a comprehensive literature search strategy in consultation with the Trials Search Coordinator of the Cochrane Menstrual Disorders and Subfertility Group (MDSG).
Two review authors (HEN and JRR) independently conducted a systematic search of the published and unpublished literature. There were no restrictions on language or publication status.
Electronic searches
We searched the following electronic databases, trial registers and websites from inception to 12 March 2015: the MDSG Specialized Register of Controlled Trials (Appendix 1), the Cochrane Central Register of Controlled Trials (Appendix 2), MEDLINE (Appendix 3), EMBASE (Appendix 4), PsycINFO (Appendix 5) and CINAHL (Appendix 6).
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in Chapter 6 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). The EMBASE search was combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (http://www.sign.ac.uk/methodology/filters.html#random).
We also searched the following electronic sources for trials:
Trial registers for ongoing and registered trials: Current Controlled Trials (www.controlled‐trials.com/), ClinicalTrials.gov, a service of the US National Institutes of Health (http://clinicaltrials.gov/ct2/home) and the World Health Organization International Clinical Trials Registry Platform search portal (WHO ICTRP) (www.who.int/trialsearch/Default.aspx).
Citation indexes (http://scientific.thomson.com/products/sci/).
Conference abstracts in the Web of Science (http://wokinfo.com/).
LILACS database, as a source of trials from the Portuguese and Spanish speaking world (http://regional.bvsalud.org/php/index.php?lang=en).
PubMed (www.ncbi.nlm.nih.gov/pubmed/), using the random control filter for PubMed from the searching chapter of the Cochrane Handbook of Systematic Reviews of Interventions.
OpenSIGLE database (http://opensigle.inist.fr/) and Google for grey literature.
NLM Gateway.
PEDro.
Australian and New Zealand Clinical Trials Registry.
Searching other resources
We handsearched the reference lists from all searched published articles for additional studies. We also contacted experts in the subject area for further references. Similarly, we handsearched the conference proceedings and abstracts not covered in the MDSG Specialized Register of Controlled Trials for relevant unpublished reports, theses and any other sources of potentially relevant references or studies, in liaison with the MDSG Trials Search Coordinator.
Data collection and analysis
Selection of studies
We developed a data screening, assessment and extraction form for the purpose of this Cochrane review, and pilot‐tested the form on a randomly selected sample of apparently applicable studies. This form included details of all relevant trial characteristics.
Two review authors (HEN and JRR) independently screened the titles and abstracts of studies identified by the searches for possible applicability in accordance with the prespecified inclusion and exclusion criteria. They discarded any studies that were clearly not applicable. We reviewed the full text of the studies identified for inclusion after this screening for eligibility according to the prespecified criteria. Where there were insufficient data to enable a decision on inclusion or exclusion, we included the trial provisionally and contacted the authors of the trial report for further information. We resolved any disagreements regarding selection at screening or eligibility by consensus or, in the event of non‐agreement, by referring to a third review author (BK or CS); no such disagreement occurred. Where the decision was to exclude a trial, we noted the reasons for this and presented this information in the 'Characteristics of excluded studies' table. For details of the selection process see also Figure 1.
1.
Study flow diagram.
Data extraction and management
Two review authors separately extracted the data from these studies using their screening, assessment and extraction form. Data extracted included demographic data (type and location of study, trial period, number of women), interventions (type of treatment protocol, dosages, method of delivery), methodology (quality assessment including method of randomisation and allocation) and outcome data (rates for each pre‐specified primary and secondary outcome). The two review authors compared the two sets of data and any disagreements would have been resolved by discussion with a third review author (BK or CS). Where data were missing or incomplete, the review authors contacted the trial authors for further details. There were no cases where studies had multiple publications. One study reported multiple interventions; we have followed the advice of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Chapter 16.5.4) and included each pairwise comparison separately for primary review outcomes.
Assessment of risk of bias in included studies
We independently assessed the risk of bias in included studies using the Cochrane 'Risk of bias' assessment tool (Higgins 2011; Appendix 8). We assessed each study under the six domains: selection bias (sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessment); attrition bias (incomplete outcome data); reporting bias (selective outcome reporting); and other bias (any 'other issues' were also considered for assessment). The 'Risk of bias' tables describe all judgements and present our conclusions; for summaries see Figure 2 and Figure 3.
2.
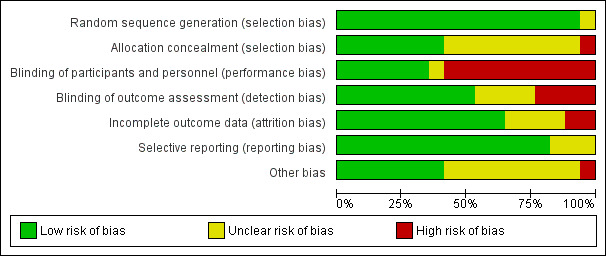
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
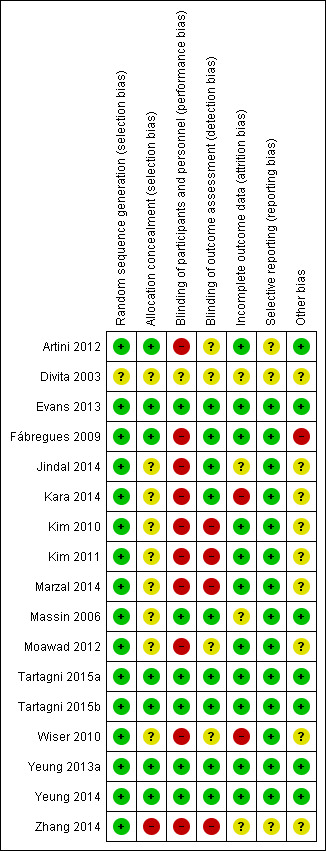
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Measures of treatment effect
For dichotomous data we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). We have presented 95% confidence intervals (CIs) for all outcomes. There were no continuous data.
Unit of analysis issues
All analyses were per woman randomised. We planned to summarise in a separate table data that did not allow valid analysis (such as data reported 'per cycle' and not 'per woman', where results might include the same woman at more than one time point or cycle) and exclude it from meta‐analyses. We would only have included first‐phase data from crossover trials.
Dealing with missing data
Where possible, we performed intention‐to‐treat (ITT) analysis. We asked trial authors via e‐mail or telephone to provide further details where reported data were insufficient or missing. Live birth or clinical pregnancy was assumed not to have occurred where these outcomes were not reported.
Assessment of heterogeneity
We assessed the characteristics of the included studies to decide whether there were sufficient similarities in participants, interventions and outcomes for meta‐analysis to be appropriate. In an initial step we conducted a visual inspection of the forest plot.
We used the I² statistic to assess heterogeneity. An I² statistic value > 50% indicated substantial heterogeneity (Higgins 2003). If this had been found, we had planned to explore this by means of sensitivity analyses as described below.
Assessment of reporting biases
We aimed to minimise publication and other biases by employing a sufficiently robust search strategy, including electronic and handsearching, grey literature including conference abstracts, registers of clinical trials, and researchers in this area of infertility research. Our review of each trial report included an evaluation of non‐ or insufficiently‐reported outcomes; where we suspected this within‐study reporting bias, we obtained the protocols where possible and compared the prespecified outcomes with those reported in the published study results.
We were alert to possible duplication bias and cross‐checked details of trial authors, locations, numbers of participants, and dates.
Data synthesis
We planned to combine results from primary studies using meta‐analysis with Review Manager (RevMan), using fixed‐effect models in comparisons as follows:
All androgens grouped by DHEA or testosterone versus placebo or no treatment.
One androgen grouped by DHEA or testosterone versus another active intervention.
An increase in the odds of a particular outcome, whether beneficial (for example, live birth) or detrimental (for example, miscarriage), is displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line.
We employed the fixed‐effect model for combined analysis in accordance with the guidelines of the Cochrane MDSG. If there were considerable variation between trials (age of participants, treatment types and dosages, outcomes reported) we planned to use the random‐effects model.
We pooled dichotomous data to calculate pooled ORs with 95% CIs.
Subgroup analysis and investigation of heterogeneity
We performed analyses to determine effects within the following subgroups:
Timing of treatment.
Previous ovarian stimulation status.
We planned to conduct analyses to determine effects within the following subgroups, but data were insufficient for any meaningful analyses:
First ART cycle.
After more than one ART cycle.
Mode of androgen delivery.
Duration of treatment.
Sensitivity analysis
We planned sensitivity analyses for the outcomes live birth and clinical pregnancy rates to explore the influence of the following factors on effect size. In conducting these analyses we would have considered whether the results would have been different if:
Eligibility had been restricted to studies judged to have low risk of bias.
A random‐effects model had been adopted.
The summary effect measure was risk ratio (RR) rather than OR.
'Summary of findings' table
We created 'Summary of findings' tables using GRADEpro Guideline Development Tool software (www.gradepro.org) to evaluate the overall quality of the body of evidence for the outcomes live birth, miscarriage, clinical pregnancy and multiple pregnancy, using GRADE working group criteria (i.e. study limitations such as risk of bias, consistency of effect, imprecision, indirectness and publication bias). Our judgements about evidence quality (high, moderate or low) were justified, documented, and incorporated into reporting of results for each outcome.
Results
Description of studies
Results of the search
The searches retrieved 931 articles. After screening titles and abstracts, 33 studies were potentially eligible and we screened the full‐text articles. We identified 16 studies that met our inclusion criteria and we excluded 16 studies due to the type of trial or of participants. We found one further eligible study by handsearching after the most recent searches on 12 March 2015. We also found seven studies that are ongoing or have not yet reported results, and two studies that had been terminated. See study tables: Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies. We have presented the screening and selection processes in Figure 1.
Included studies
Study design and setting
Seventeen studies met the inclusion criteria. Twelve were trials of DHEA (Artini 2012; Divita 2003; Evans 2013; Jindal 2014; Kara 2014; Moawad 2012; Tartagni 2015a; Tartagni 2015b; Wiser 2010; Yeung 2013a; Yeung 2014; Zhang 2014) and five were testosterone trials (Fábregues 2009; Kim 2010; Kim 2011; Marzal 2014; Massin 2006). All were RCTs and were based in single‐unit fertility clinics in Argentina, China, France, Hong Kong, India, Israel, Italy, Korea, Spain, Turkey, UAE and UK. Fifteen studies were two‐group parallel trials (Artini 2012; Divita 2003; Evans 2013; Fábregues 2009; Jindal 2014; Kara 2014; Kim 2011; Massin 2006; Moawad 2012; Tartagni 2015a; Tartagni 2015b; Wiser 2010; Yeung 2013a; Yeung 2014; Zhang 2014) while Marzal 2014 and Kim 2010 were multi‐arm trials.
Participants
Of the 17 studies, 828 women were in the intervention group and 785 in the control group. In all but two studies (Tartagni 2015a; Yeung 2013a), participants were stated to be women with infertility of greater than one year. The mean age of participants across the studies ranged from 30 to 40 years. We were unable to obtain further information from the authors of one study (Divita 2003). Apart from Tartagni 2015a and Yeung 2013a, all the women were defined as having responded poorly to previous ovarian stimulation, although the definition of poor response was different in each study. We have provided the full details of the inclusion and exclusion criteria for each study in the Characteristics of included studies tables. The common exclusion criterion was age of participants at time of participation.
Interventions
DHEA (12 studies):
Six studies compared DHEA with placebo (Divita 2003; Evans 2013; Tartagni 2015a; Tartagni 2015b; Yeung 2013a; Yeung 2014).
Six studies compared DHEA with no treatment (Artini 2012; Jindal 2014; Kara 2014; Moawad 2012; Wiser 2010; Zhang 2014).
The dosage varied: one study used a daily oral dose of 40 mg of micronised DHEA sulfate (DHEAS) as co‐treatment with GnRHa (commenced in the mid‐luteal phase of the previous cycle) (Divita 2003), while most used a daily oral dose of 75 mg DHEA as a pre‐ and then co‐treatment with a long gonadotropin‐releasing hormone agonist (GnRHa) protocol (Artini 2012; Evans 2013; Kara 2014; Moawad 2012; Tartagni 2015a; Tartagni 2015b; Wiser 2010; Yeung 2013a; Yeung 2014; Zhang 2014). Wiser 2010 included two IVF cycles. Jindal 2014 used a 75 mg dose of DHEA in a combination of GnRHa and antagonist cycles.
Testosterone (five studies):
One study compared transdermal testosterone with placebo gel (Massin 2006).
Three studies compared transdermal testosterone with no treatment (Fábregues 2009; Kim 2010; Kim 2011).
One study compared transdermal testosterone with estradiol and with estradiol plus oral contraceptive pill (Marzal 2014).
Again, the dosage and length of treatment varied: 2.5 mg per day pre‐treatment (20 μg/kg) for five days (Fábregues 2009); 10 mg per day pre‐treatment for 15 to 20 days (Massin 2006); 12.5 mg per day pre‐treatment for 14, 21 or 28 days (Kim 2010); 12.5 mg per day pre‐treatment for 21 days (Kim 2011); and 20 μg/kg per day for six days (Marzal 2014).
Massin 2006 and Marzal 2014 utilised GnRH agonist protocols, while Kim 2010 and Kim 2011 both used a GnRH antagonist multiple‐dose protocol (MDP).
One study of transdermal testosterone used a standard long GnRH analogue protocol in the treatment group, but employed a minidose GnRHa protocol in the control group (Fábregues 2009).
Outcomes
Primary outcomes
The primary outcomes for this Cochrane review were live birth and miscarriage.
Thirteen studies (Evans 2013; Fábregues 2009; Jindal 2014; Kim 2010; Kim 2011; Marzal 2014; Massin 2006; Moawad 2012; Tartagni 2015a; Tartagni 2015b; Wiser 2010; Yeung 2013a; Yeung 2014) reported on live birth, eight evaluating DHEA and five evaluating testosterone. This includes two trials reporting ongoing pregnancy (Moawad 2012; Yeung 2013a).
Thirteen studies reported on miscarriage (Evans 2013; Fábregues 2009; Jindal 2014; Kim 2010; Kim 2011; Marzal 2014; Massin 2006; Moawad 2012; Tartagni 2015a; Tartagni 2015b; Yeung 2013a; Yeung 2014; Zhang 2014), eight evaluating DHEA and five testosterone. A further study (Wiser 2010) investigating DHEA reported miscarriage rate only as a total after both treatment cycles so we could not include those data in the analyses.
Secondary outcomes
The secondary outcomes were:
Clinical pregnancy rate per woman randomised, reported in 17 studies: 12 in the DHEA comparison (Artini 2012; Divita 2003; Evans 2013; Jindal 2014; Kara 2014; Moawad 2012; Tartagni 2015a; Tartagni 2015b; Wiser 2010; Yeung 2013a; Yeung 2014; Zhang 2014) and five in the testosterone comparisons (Fábregues 2009; Kim 2010; Kim 2011; Marzal 2014; Massin 2006).
Multiple pregnancy rate, reported in eight studies. Five studies were in the DHEA comparison (Tartagni 2015a; Tartagni 2015b; Wiser 2010; Yeung 2014) and three in the testosterone comparison (Fábregues 2009; Kim 2010; Kim 2011).
Adverse effects to the woman. Four studies collected data on adverse events: (Artini 2012; Yeung 2013a; Yeung 2014; Zhang 2014).
Adverse fetal effects including fetal anomalies. None of the studies reported on this outcome.
Excluded studies
We excluded 16 studies. Four studies were not RCTs (Hyman 2013; Motta 2006; Singh 2013; Sönmezer 2009). Another was a case series (Casson 2000); there were three case‐control studies (Barad 2006; Barad 2007; de los Santos 2013); two were cohort studies (Fusi 2013; Gleicher 2013); one was a non‐randomised self controlled clinical trial (Balasch 2006); one had no randomised control group (Monterde 2013); and the participants of two studies did not meet our inclusion criteria (Sipe 2010; Yeung 2013b). Two studies were stopped early with no data available (Barad 2008b; Fábregues 2011).
Risk of bias in included studies
Allocation
Random sequence generation
Sixteen studies were at low risk of this bias as randomisation was either done by a computer or generated by a random permutation table (Artini 2012; Evans 2013; Fábregues 2009; Jindal 2014; Kara 2014; Kim 2010; Kim 2011; Marzal 2014; Massin 2006; Moawad 2012; Tartagni 2015a; Tartagni 2015b; Wiser 2010; Yeung 2013a; Yeung 2014; Zhang 2014). In the remaining study, insufficient information was available to determine the method of sequence generation (Divita 2003).
Allocation concealment
Seven studies had a low risk of this bias as concealment was achieved using sealed, opaque, sequential envelopes or was otherwise satisfactory (Artini 2012; Evans 2013; Fábregues 2009; Tartagni 2015a; Tartagni 2015b; Yeung 2013a; Yeung 2014). Nine studies were judged to have an unclear risk of bias for allocation concealment as there was no or insufficient information (Divita 2003; Jindal 2014; Kara 2014; Kim 2010; Kim 2011; Marzal 2014; Massin 2006; Moawad 2012; Wiser 2010). Of these, two reported that they had used sealed envelopes to conceal the allocation (Kim 2011; Wiser 2010) but numbering and opacity detail was not stated. One study was at high risk of this bias as no blinding was attempted (Zhang 2014).
Blinding
Performance bias
In seven studies the participants were blinded as a placebo was used: placebo gel for testosterone gel (Massin 2006) or placebo instead of DHEA (Evans 2013; Tartagni 2015a; Tartagni 2015b; Yeung 2013a; Yeung 2014). However, one study had insufficient information to assess the blinding of study personnel so was judged at unclear risk of this bias (Divita 2003). In 10 studies participants were not blinded as those in the control group received no treatment (Artini 2012; Fábregues 2009; Jindal 2014; Kara 2014; Kim 2010; Kim 2011; Marzal 2014; Moawad 2012; Wiser 2010; Zhang 2014).
Detection bias
Nine studies stated that the outcome assessors were blinded to the patients' treatment allocation (Evans 2013; Fábregues 2009; Jindal 2014; Kara 2014; Massin 2006; Tartagni 2015a; Tartagni 2015b; Yeung 2013a; Yeung 2014). In four studies, it was unclear whether outcome assessors were blinded (Artini 2012; Divita 2003; Moawad 2012; Wiser 2010). Four studies stated that the clinicians were not blinded (Kim 2010; Kim 2011; Marzal 2014; Zhang 2014) so we assessed these studies as at high risk of bias.
Incomplete outcome data
In 16 studies all or most of the women randomised in each study were analysed. Fourteen studies reported drop‐out rates (Artini 2012; Divita 2003; Evans 2013; Fábregues 2009; Kim 2010; Kim 2011; Marzal 2014; Massin 2006; Tartagni 2015a; Tartagni 2015b; Wiser 2010; Yeung 2013a; Yeung 2014; Zhang 2014). Wiser 2010 had high dropout rates in both intervention and control groups. Zhang 2014 lost nine women to follow‐up, all in the treatment group, for a variety of reasons. Moawad 2012 analysed all randomised women but it is unclear how many women dropped out of the study. Kara 2014 excluded some participants after randomisation. We have no information about attrition rates for Jindal 2014. Drop out rates were nil or one only in 12 studies.
Selective reporting
In 14 studies, we assessed the risk of reporting bias as low, as they reported outcomes relevant to this Cochrane review (Evans 2013; Fábregues 2009; Jindal 2014 ; Kara 2014; Kim 2010; Kim 2011; Marzal 2014; Massin 2006; Moawad 2012; Tartagni 2015a; Tartagni 2015b; Wiser 2010; Yeung 2013a; Yeung 2014). For three studies, information was insufficient, and these studies were at unclear risk of bias (Artini 2012; Divita 2003; Zhang 2014).
Other potential sources of bias
Fábregues 2009 used different protocols for ovarian suppression in the control and study arms, potentially introducing bias to the study. We did not identify any other sources of bias in seven studies (Artini 2012; Evans 2013; Massin 2006; Tartagni 2015a; Tartagni 2015b; Yeung 2013a; Yeung 2014), while in nine studies information was insufficient to make a judgement so were at unclear risk of bias (Divita 2003; Jindal 2014; Kara 2014; Kim 2010; Kim 2011; Marzal 2014; Moawad 2012; Wiser 2010).
Effects of interventions
1 DHEA or testosterone versus placebo/no treatment
We have not pooled the results for DHEA and testosterone in this comparison as the interventions are similar but clinically distinct.
Primary outcomes:
1.1 Live birth/ongoing pregnancy rate
DHEA versus placebo/no treatment
Six studies reported on live birth rates (Evans 2013; Jindal 2014; Tartagni 2015; Tartagni 2015b; Wiser 2010; Yeung 2014). Two trials reported ongoing pregnancy (Moawad 2012; Yeung 2013a), and we have combined these with the live birth results in a composite outcome. Pre‐treatment with DHEA was associated with improved live birth/ongoing pregnancy rates (OR 1.81, 95% CI 1.25 to 2.62; eight RCTs, N = 878, I² statistic = 14%, moderate quality evidence; Analysis 1.1; Figure 4). This suggests that in women with an 12% chance of live birth with placebo or no treatment, the live birth rate in women using DHEA will be between 14% and 26%.
4.
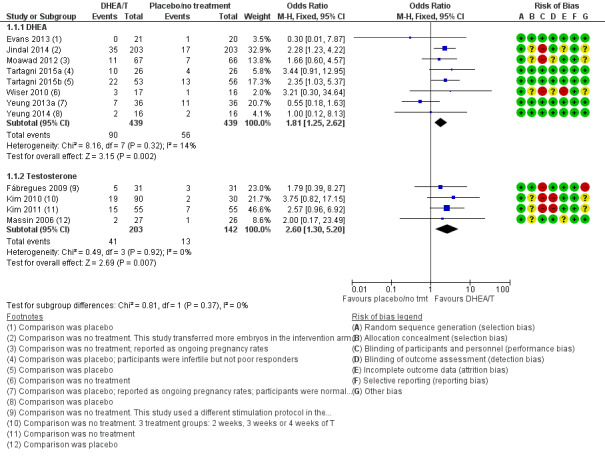
Forest plot of comparison: 1 DHEA or testosterone versus placebo/no treatment, outcome: 1.1 Live birth/ongoing pregnancy rate.
There was no evidence of a difference between the groups in a sensitivity analysis removing all studies at high risk of performance bias (OR 1.50, 95% CI 0.88 to 2.56; five RCTs, N = 306, I² statistic = 43%).
A sensitivity analysis removing the two studies reporting only ongoing pregnancy did not change the OR significantly (OR 12.24, 95% CI 1.45 to 3.46; six RCTs, N = 673).
Analysis 1.2 presents live birth rates subgrouped by previous ovarian stimulation status. Two trials were in treatment‐naive women, showing no evidence of treatment effect (OR 1.18, 95% CI 0.53 to 2.60, two RCTs, N = 124, Analysis 1.6.1). Among the women who had had previous fertility treatment, DHEA was more effective than placebo or no treatment (OR 2.04, 95% CI 1.34 to 3.10, six RCTs, N = 754, Analysis 1.6.2).
1.2. Analysis.
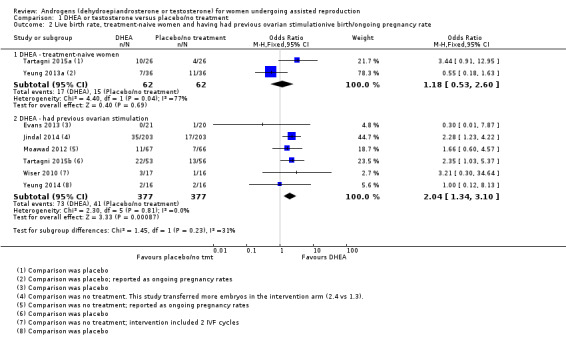
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 2 Live birth rate, treatment‐naive women and having had previous ovarian stimulationive birth/ongoing pregnancy rate.
We did not perform planned subgroup analyses by dose and mode of delivery. The included studies for DHEA were based on a similar dose taken orally.
Length of treatment with DHEA varied from two to 26 weeks, and all trials for which information was available continued administration of DHEA until the end of ovarian stimulation. See Analysis 1.3 presenting studies subgrouped by length of treatment. Data were insufficient for us to draw any conclusions on an optimal length of administration.
1.3. Analysis.
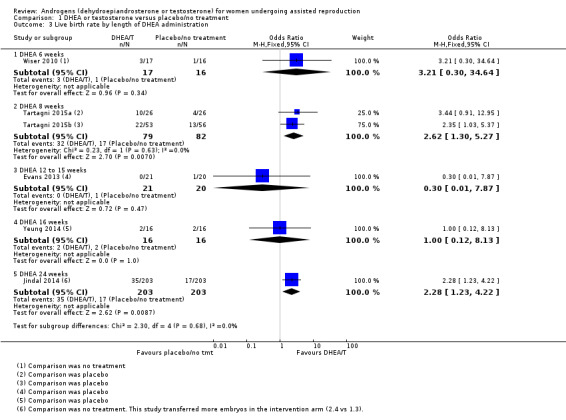
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 3 Live birth rate by length of DHEA administration.
Testosterone versus placebo/no treatment
Four studies reported on live birth rates (Fabregues 2009; Kim 2010; Kim 2011, Massin 2006) and found that pre‐treatment with T was associated with improved live birth rates (OR 2.60, 95% CI 1.30 to 5.20; four RCTs, N = 345, I² statistic = 0%, moderate quality evidence; Analysis 1.1; Figure 4). This suggests that in women with an 8% chance of live birth with placebo or no treatment, the live birth rate in women using T will be between 12% and 32%. Fabregues 2009 used different ovarian suppression protocols in the study and control arms. A sensitivity analysis removing this study increased the CI, but did not change the outcome with regard to live birth (OR 2.84, 95% CI 1.30 to 6.21; three RCTs, N = 283, I² statistic = 0%).
The event rates reported by Kim 2010 and Kim 2011 were high, and we assessed these studies as at high risk for performance and detection bias. We tested this in a sensitivity analysis by removing both studies, and this reduced the effect size (OR 1.85, 95% CI 0.51 to 6.78; N = 115, I² statistic = 0%). In a further sensitivity analysis removing all studies at high risk of performance bias there was no evidence of an association between pre‐treatment with T and live birth rates in the remaining study (OR 2.00, 95% CI 0.17 to 23.49; one RCT, N = 53, I² statistic = 0%).
All the testosterone studies were in women who had had previous fertility treatment. We did not perform planned subgroup analyses by dose and mode and length of delivery. In the testosterone studies reporting this outcome three used transdermal gel with two different doses and the other used transdermal patches. Length of treatment with T varied from five to 28 days. In all the studies T was administered up until the start of ovarian stimulation. See Analysis 1.4 presenting studies subgrouped by length of treatment. Data do not allow us to draw any conclusions on an optimal length of administration.
1.4. Analysis.
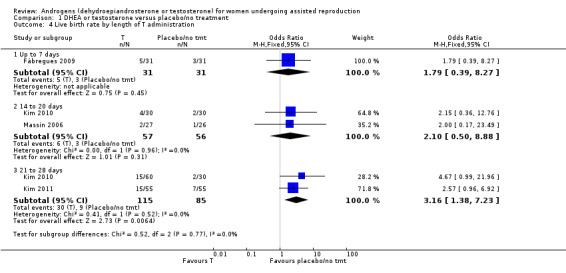
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 4 Live birth rate by length of T administration.
1.2 Miscarriage rate
DHEA versus placebo/no treatment
Eight studies reported this outcome (Evans 2013; Jindal 2014; Moawad 2012; Tartagni 2015; Tartagni 2015b; Yeung 2013a; Yeung 2014; Zhang 2014). There was no evidence of a difference between the intervention and control groups (OR 0.58, 95% CI 0.29 to 1.17; eight RCTs, N = 950, I² statistic = 0%, moderate quality evidence; Analysis 1.5; Figure 5).
1.5. Analysis.
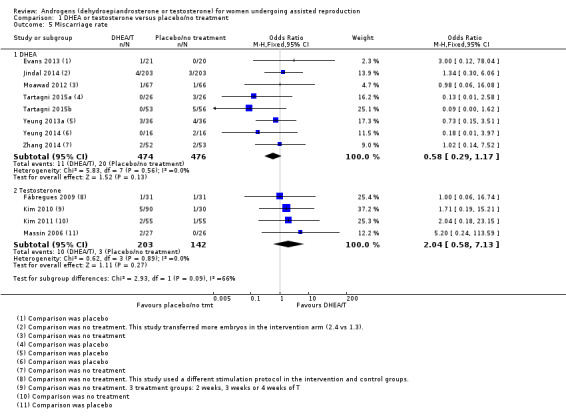
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 5 Miscarriage rate.
5.
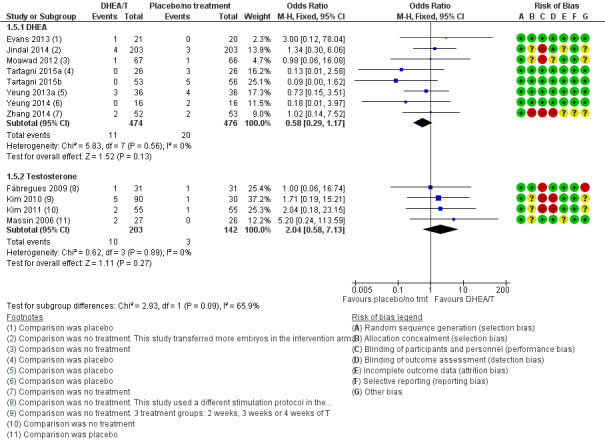
Forest plot of comparison: 1 DHEA or testosterone versus placebo/no treatment, outcome: 1.5 Miscarriage rate.
Analysis 1.6 presents miscarriage rates subgrouped by previous ovarian stimulation status. Two trials were in treatment‐naive women, showing no evidence of treatment effect (OR 0.44, 95% CI 0.12 to 1.63, two RCTs, N = 124, Analysis 1.8.1). Among the women who had had previous fertility treatment, there was no evidence of a difference between DHEA and placebo or no treatment (OR 0.65, 95% CI 0.29 to 1.50, six RCTs, N = 826, Analysis 1.6.2).
1.6. Analysis.
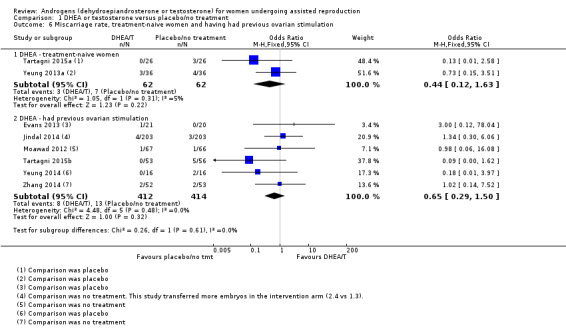
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 6 Miscarriage rate, treatment‐naive women and having had previous ovarian stimulation.
We did not perform planned subgroup analyses by dose and mode of delivery. The included studies for DHEA were based on a similar dose taken orally.
Length of treatment with DHEA varied from two to 26 weeks, and all trials for which information was available continued administration of DHEA until the end of ovarian stimulation. See Analysis 1.7 presenting studies subgrouped by length of treatment. Data were insufficient for us to draw any conclusions on an optimal length of administration.
1.7. Analysis.
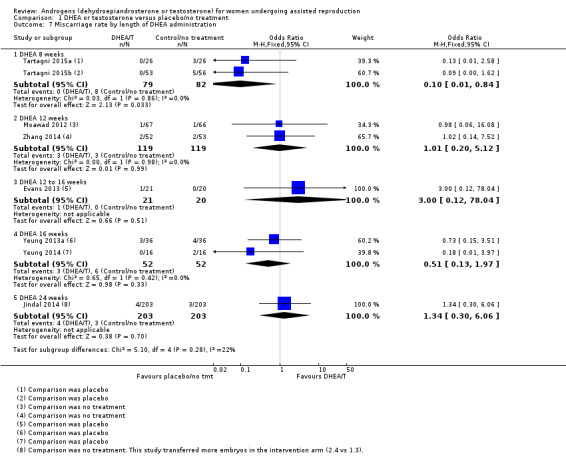
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 7 Miscarriage rate by length of DHEA administration.
Testosterone versus placebo/no treatment
Four studies reported this outcome (Fabregues 2009; Kim 2010; Kim 2011; Massin 2006) and found no evidence of a difference between the intervention and the control groups (OR 2.04, 95% CI 0.58 to 7.13; four RCTs, N = 345, I² statistic = 0%, low quality evidence; Analysis 1.5; Figure 5).
Analysis 1.8 presents the studies subgrouped by length of treatment.
1.8. Analysis.
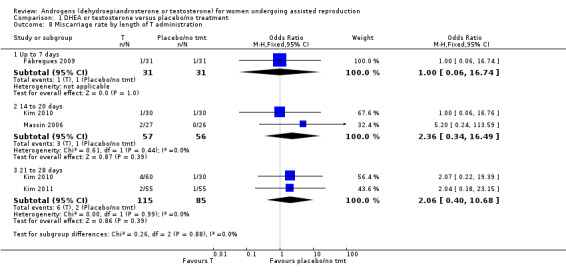
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 8 Miscarriage rate by length of T administration.
Secondary outcomes:
1.3 Clinical pregnancy rate
DHEA versus placebo/no treatment
Twelve studies reported on this outcome (Artini 2012; Divita 2003; Evans 2013; Jindal 2014; Kara 2014; Moawad 2012; Tartagni 2015; Tartagni 2015b; Wiser 2010; Yeung 2013a; Yeung 2014; Zhang 2014) and found that pre‐treatment with DHEA was associated with an increase in clinical pregnancy rates (OR 1.34, 95% CI 1.01 to 1.76; 12 RCTs, N = 1246, I² statistic = 0%, moderate quality evidence; Analysis 1.9; Figure 6). The evidence suggests that in women with an 21% chance of clinical pregnancy with placebo or no treatment, the clinical pregnancy rate in women using DHEA will be between 21% and 32%.
1.9. Analysis.
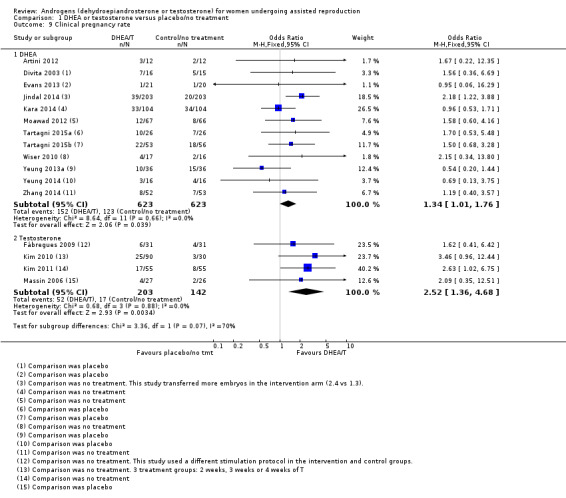
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 9 Clinical pregnancy rate.
6.
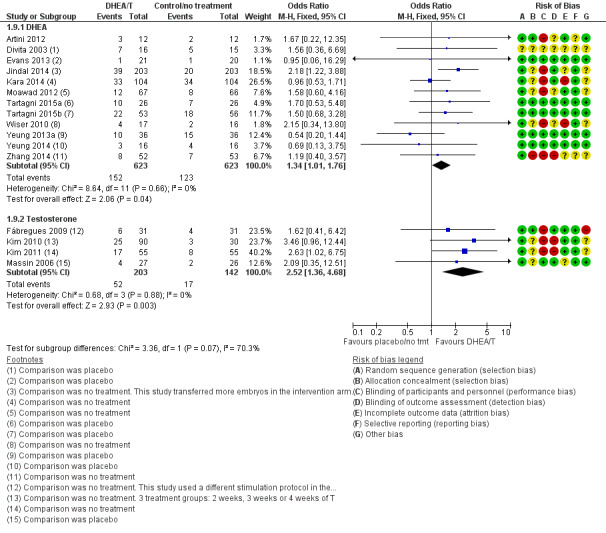
Forest plot of comparison: 1 DHEA or testosterone versus placebo/no treatment, outcome: 1.9 Clinical pregnancy rate.
Two included studies tested the use of DHEA versus placebo in women not identified as poor responders (Tartagni 2015; Yeung 2013a). Tartagni 2015 described their participants as infertile but not poor responders; the women had not had any previous assisted reproductive interventions. The participants in Yeung 2013a were normal responders; it reported clinical pregnancy rates and found no evidence of a difference between the groups. With removal of these studies in a sensitivity analysis the evidence of an association between pre‐treatment with DHEA and clinical pregnancy rates remained (OR 1.44, 95% CI 1.06 to 1.94; 10 RCTs, N = 1122, I² statistic = 0%).
In a further sensitivity analysis removing the studies at high risk of performance bias there was no evidence of an association between pre‐treatment with DHEA and clinical pregnancy rates (OR 1.11, 95% CI 0.69 to 1.79; six RCTs, N = 337, I² statistic = 0%).
Testosterone versus placebo/no treatment
Four studies reported on this outcome (Fabregues 2009; Kim 2010; Kim 2011; Massin 2006). We found an association between pre‐treatment with T and increased clinical pregnancy rates (OR 2.52, 95% CI 1.36 to 4.68; four RCTs, N = 345, I² statistic = 0%, moderate quality evidence; Analysis 1.3; Figure 6). This suggests that in women with an 12% chance of clinical pregnancy with placebo or no treatment, the clinical pregnancy rate in women using DHEA will be between 16% and 38%. Fabregues 2009 used different ovarian suppression protocols in the study and control arms. A sensitivity analysis removing this study increased the CI (OR 2.80, 95% CI 1.39 to 5.61; three RCTs, N = 283). In a further sensitivity analysis removing all studies at high risk of performance bias there is no evidence of an association between pre‐treatment with T and clinical pregnancy rates in the remaining study (OR 2.09, 95% CI 0.35 to 12.51, one RCT, N = 53).
1.4 Multiple pregnancy
DHEA versus placebo/no treatment
Five studies reported on this outcome (Evans 2013; Tartagni 2015; Tartagni 2015b; Wiser 2010; Yeung 2014) with only one multiple pregnancy, in the DHEA group (OR 3.23, 95% CI 0.13 to 81.01; five RCTs, N = 267, very low quality evidence; Analysis 1.10). We were unable to obtain data from authors of the other trials.
1.10. Analysis.
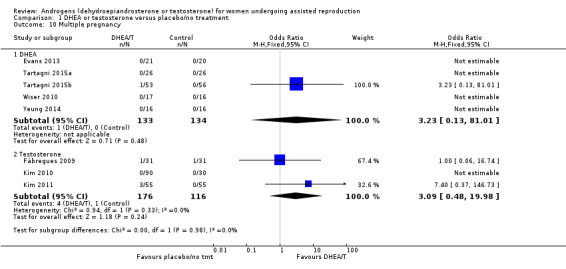
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 10 Multiple pregnancy.
Testosterone versus placebo/no treatment
Three studies reported on this outcome (Fabregues 2009; Kim 2010; Kim 2011), with four events in the testosterone group and one in the placebo/no treatment group (OR 3.09, 95% CI 0.48 to 19.98; three RCTs, N = 292, very low quality evidence; Analysis 1.10). We were unable to obtain data from the author of the remaining trial.
1.5 Adverse effects to the woman
DHEA versus placebo/no treatment
1.5.1 Increased acne
Two studies reported this outcome (Yeung 2014; Zhang 2014): both studies reported events in the DHEA groups and not in the controls (OR 5.40, 95% CI 0.61 to 47.63; two RCTs, N = 137, I² statistic = 0%; Analysis 1.11), but event numbers were low.
1.11. Analysis.
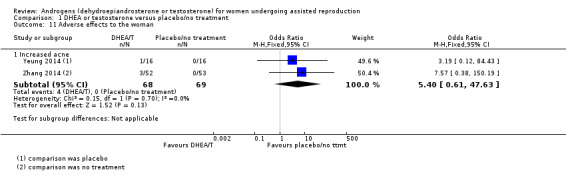
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 11 Adverse effects to the woman.
Yeung 2013a reported that up to 22% of participants in the DHEA group complained of increased acne, compared with 8.3% in the placebo group, and another study noted that some women reported increased sebum production and a few developed transitional hirsutism, but gave no data (Artini 2012).
1.5.2 Other adverse events
Zhang 2014 reported that one woman complained of dizziness, but did not state whether she was in the intervention or the control group. All other studies recorded in narrative that they had not noted any adverse effects in any of their participants.
Testosterone versus placebo/no treatment
None of the testosterone studies reported any adverse effects.
1.6 Adverse fetal effects such as fetal anomalies
DHEA or testosterone versus placebo/no treatment
None of the included studies reported on this outcome.
Publication bias
Visual scanning of a funnel plot for the outcome with most data (DHEA or testosterone versus placebo/no treatment: clinical pregnancy rate; dichotomous data) suggests no obvious sign of publication bias (Figure 7). The studies presented in the funnel plot are for both DHEA and T; these were not pooled in the analyses.
7.
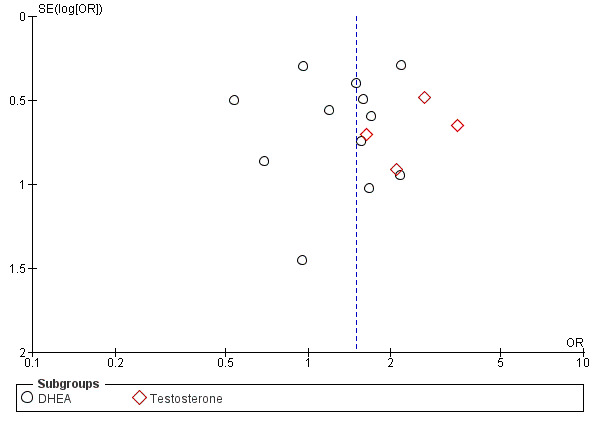
Funnel plot of comparison: 1 DHEA or testosterone versus placebo/no treatment, outcome: 1.9 Clinical pregnancy rate.
2. Testosterone versus another treatment (estradiol)
One study reported this comparison: some results for Marzal 2014 were supplied by the study author.
Primary outcomes:
2.1 Live birth rate
2.1.1 Testosterone versus estradiol
In a comparison with estradiol, Marzal 2014 reported no evidence of an effect of testosterone on live birth rate (OR 0.10, 95% CI ‐0.12 to 0.32; one RCT, N = 46; Analysis 2.1).
2.1. Analysis.
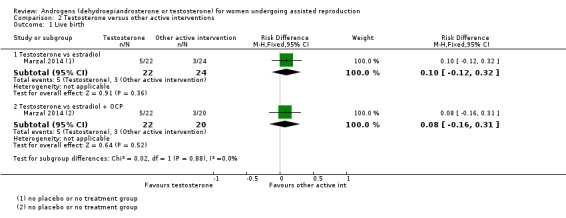
Comparison 2 Testosterone versus other active interventions, Outcome 1 Live birth.
2.2 Miscarriage rate
2.2.1 Testosterone versus estradiol
In a comparison with estradiol, Marzal 2014 reported no evidence of an effect of testosterone on miscarriage rate (OR 0.70, 95% CI 0.11 to 4.64; one RCT, N = 46; Analysis 2.2).
2.2. Analysis.
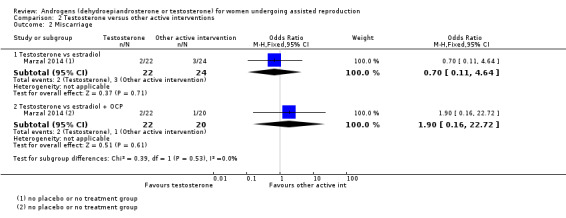
Comparison 2 Testosterone versus other active interventions, Outcome 2 Miscarriage.
Secondary outcomes:
2.3 Clinical pregnancy rate
2.3.1 Testosterone versus estradiol
In a comparison with estradiol, Marzal 2014 reported no evidence of an effect of testosterone on clinical pregnancy rate (OR 1.40, 95% CI 0.39 to 5.07, one RCT, N = 46; Analysis 2.3).
2.3. Analysis.
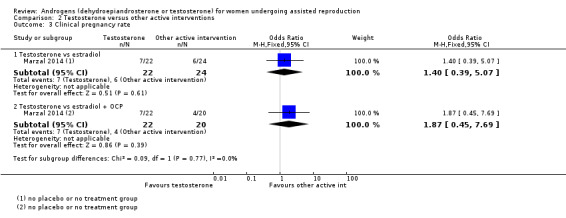
Comparison 2 Testosterone versus other active interventions, Outcome 3 Clinical pregnancy rate.
2.4 Multiple pregnancy rate
This outcome was not reported.
2.5 Adverse effects to the woman
2.5.1 Testosterone versus estradiol
This outcome was not reported.
2.6 Adverse fetal effects such as fetal anomalies
2.6.1 Testosterone versus estradiol
This outcome was not reported.
Discussion
Summary of main results
This Cochrane review evaluated the effectiveness and safety of using DHEA or testosterone as adjuncts to ART in women undergoing assisted reproduction. Twelve trials compared DHEA with placebo or no treatment, four compared testosterone with placebo or no treatment, and one compared testosterone with estradiol. All but two of the included studies had study populations of women identified as poor responders. Apart from two larger studies, the number of women in the trials was generally small.
Treatment with DHEA and testosterone appeared to improve the live birth/ongoing pregnancy rate and clinical pregnancy rate when given to women identified as poor responders. However the treatment effect for DHEA and T no longer reached significance when we excluded studies at high risk of bias in sensitivity analyses.
Length of treatment with DHEA varied from two to 26 weeks, and with T from five to 28 days.
Evidence for the safety of DHEA and testosterone was scarce. In the 12 studies reporting on miscarriage we found no evidence of a difference in miscarriage rates between the groups. There were multiple pregnancies in three of the eight studies that reported this outcome. In all of the included studies, it was stated in narrative that any observed adverse effects did not differ between treatment and control groups. This was not well reported, and more specific detail would have been desirable.
The quality of evidence in the included studies was generally moderate, the main limitations being lack of blinding, imprecision and poor reporting of study methods.
Overall completeness and applicability of evidence
This Cochrane review included 17 studies with data that were relevant to this review question. The study populations were broadly similar in terms of age. All participants in the included studies were defined as poor responders to previous ovarian stimulation, with the exception of two studies (one included normal responders, and participants in the other were described as subfertile but not poor responders). Thirteen studies reported on the primary outcomes of live birth and miscarriage. Given the study populations, the results of this Cochrane review will be largely applicable to women who have been identified as poor responders.
Quality of the evidence
The methodological quality of the included trials varied. In nine trials the comparator was no treatment, although blinding with placebo was possible. Only seven studies reported adequate methods of both randomisation and allocation concealment. One study used different downregulation protocols in the treatment and control arms, limiting its applicability. See Figure 2 and Figure 3 for our 'Risk of bias' assessments of the included studies. A number of studies lacked relevant detail on some 'Risk of bias' domains including allocation concealment.
Overall study quality was moderate. Some studies were conducted to a high standard. The reasons for downgrading the quality of the evidence were lack of blinding and imprecision, with low numbers of events and poor reporting of study methods. See Table 1 and Table 2 for more details.
Potential biases in the review process
We made every effort to identify all eligible studies. Not all trial authors responded to our requests for additional information but we have no way of knowing whether our requests always reached the relevant person.
Agreements and disagreements with other studies or reviews
A review of non‐randomised and randomised studies of androgen adjuvants (testosterone and DHEA) (Sunkara 2011) found no evidence for efficacy in improving live birth rates. Their review included three studies also in this Cochrane review (Fábregues 2009; Massin 2006; Wiser 2010).
However Venetis 2011, a systematic review of RCTs evaluating transdermal testosterone in poor responders, found that pre‐treatment with testosterone was associated with a significant increase in the probability of clinical pregnancy and live birth. This review had two included studies (this was a conference abstract and the studies were not named).
Another review of studies evaluating the administration of testosterone, DHEA, aromatase inhibitors, recombinant luteinizing hormone (rLH) and recombinant human chorionic gonadotrophin (rhCG) before or during ovarian stimulation of poor responders (Bosdou 2012) reported an increase in clinical pregnancy and live birth rates after pretreatment with transdermal testosterone, but no difference in rates between those who received DHEA and those who did not. Bosdou 2012 included the following studies that are also included in this Cochrane review: Kim 2011; Massin 2006; and Wiser 2010.
Another systematic review published in 2012, González‐Comadran 2012, examined the evidence for the use of transdermal testosterone in poor responders undergoing IVF. It included Fábregues 2009; Kim 2011; and Massin 2006, which are also included in this Cochrane review, and concluded that there is evidence to support the use of transdermal testosterone in women who are considered poor responders. However the authors note that it is still unclear which exact subgroup of those women would benefit from this treatment, and that small study numbers suggest results should be interpreted with caution.
Authors' conclusions
Implications for practice.
There is evidence that in women identified as 'poor responders' undergoing ART, pre‐treatment with DHEA or T may be associated with improved live birth rates, based on moderate quality evidence. There is insufficient evidence to draw conclusions about the safety of either androgen. Data on adverse events were sparse, but those events reported were of minor concern. Definitive conclusions regarding the clinical role of either androgen awaits evidence from further well designed studies. DHEA and testosterone could be used with caution for women described as 'poor responders' until robust evidence is available from further completed studies.
Implications for research.
Future larger and well‐conducted RCTs of these androgens are needed to confirm whether either pre‐ or co‐treatment is effective as an adjunct to the IVF process. In particular, investigators need to recruit an adequate number of participants for meaningful analysis to be possible. More complete documentation of the methods employed for random sequence generation and allocation concealment, and for performance and detection bias is desirable. Study protocols should provide for reporting of live births and adverse effects. CONSORT 2010 details all relevant standards.
The evidence to date is limited by generally small sample sizes and inadequately reported study methodology. Participants so far, apart from two studies, have been women identified as having responded poorly to previous ovarian stimulation.
Acknowledgements
We acknowledge the assistance of the Cochrane MDSG, especially Marian Showell, Trials Search Coordinator. In addition, we thank the authors of Artini 2012; Marzal 2014; Evans 2013, Fábregues 2009, Kim 2010; Kim 2011, Massin 2006, Yeung 2013b; Yeung 2013a; Yeung 2014 and Zhang 2014 for further information supplied in response to our queries.
Appendices
Appendix 1. MDSG search strategy
Keywords CONTAINS "IUI" or "Intrauterine Insemination" or "insemination" or "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "*Ovulation Induction" or "ovarian hyperstimulation syndrome" or Title CONTAINS "IUI" or "Intrauterine Insemination" or "insemination" or "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "*Ovulation Induction" or "subfertility "or"subfertility‐female"or "infertile"or"infertility "
AND
Keywords CONTAINS "androgens"or "androgen"or androstenedione"or"*Testosterone"or"DHEA"or "DHEAS"or "dehydroepiandrosterone"or "Prasterone" or Title CONTAINS "androgens"or "androgen"or "androstenedione"or"*Testosterone"or"DHEA"or "DHEAS"or "dehydroepiandrosterone"or "Prasterone"
Appendix 2. CENTRAL search strategy
This search was first carried out on 5 July 2012, and updated on 17 December 2013, 22 May 2014 and 12 March 2015.
1 androgens/ or exp testosterone/ or exp dehydroepiandrosterone/ (2419) 2 exp Dehydroepiandrosterone Sulfate/ (240) 3 DHEA$.tw. (536) 4 dehydroepiandrosterone.tw. (606) 5 testosterone.tw. (2738) 6 prasterone$.tw. (15) 7 Androstenolone.tw. (3) 8 Dehydroisoandrosterone.tw. (0) 9 or/1‐8 (3595) 10 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (1625) 11 embryo transfer$.tw. (925) 12 vitro fertili?ation.tw. (1372) 13 ivf‐et.tw. (263) 14 ivf.tw. (1989) 15 icsi.tw. (719) 16 intracytoplasmic sperm injection$.tw. (435) 17 (blastocyst adj2 transfer$).tw. (79) 18 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp ovulation induction/ (2264) 19 assisted reproduct$.tw. (404) 20 artificial insemination.tw. (54) 21 iui.tw. (304) 22 intrauterine insemination$.tw. (412) 23 ovulation induc$.tw. (472) 24 (ovari$ adj2 stimulat$).tw. (779) 25 superovulat$.tw. (136) 26 ovarian hyperstimulation.tw. (558) 27 COH.tw. (124) 28 infertil$.tw. (1873) 29 subfertil$.tw. (138) 30 (ovari$ adj2 induction).tw. (27) 31 or/10‐30 (5515) 32 9 and 31 (208)
Appendix 3. MEDLINE search strategy
This search was first carried out on 5 July 2012, and updated on 17 December 2013, 22 May 2014 and 12 March 2015.
1 androgens/ or exp testosterone/ or exp dehydroepiandrosterone/ (85573) 2 exp Dehydroepiandrosterone Sulfate/ (3517) 3 DHEA$.tw. (6760) 4 dehydroepiandrosterone.tw. (9990) 5 testosterone.tw. (64761) 6 prasterone$.tw. (87) 7 Androstenolone.tw. (61) 8 Dehydroisoandrosterone.tw. (220) 9 or/1‐8 (106503) 10 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (33627) 11 embryo transfer$.tw. (8461) 12 vitro fertili?ation.tw. (17218) 13 ivf‐et.tw. (1927) 14 ivf.tw. (16972) 15 icsi.tw. (5831) 16 intracytoplasmic sperm injection$.tw. (5137) 17 (blastocyst adj2 transfer$).tw. (580) 18 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp ovulation induction/ (54281) 19 assisted reproduct$.tw. (9272) 20 artificial insemination.tw. (4901) 21 iui.tw. (1227) 22 intrauterine insemination$.tw. (1850) 23 ovulation induc$.tw. (3488) 24 (ovari$ adj2 stimulat$).tw. (5144) 25 superovulat$.tw. (2976) 26 ovarian hyperstimulation.tw. (4019) 27 COH.tw. (1189) 28 infertil$.tw. (43391) 29 subfertil$.tw. (3864) 30 (ovari$ adj2 induction).tw. (238) 31 or/10‐30 (102280) 32 randomized controlled trial.pt. (391739) 33 controlled clinical trial.pt. (90143) 34 randomized.ab. (308850) 35 placebo.tw. (169314) 36 clinical trials as topic.sh. (175823) 37 randomly.ab. (218392) 38 trial.ti. (133182) 39 (crossover or cross‐over or cross over).tw. (63503) 40 or/32‐39 (962105) 41 exp animals/ not humans.sh. (4067794) 42 40 not 41 (888111) 43 9 and 31 and 42 (310) 44 (201211$ or 201212$).ed. (155436) 45 2013$.ed. or 2013$.dp. (1357105) 46 44 or 45 (1512127) 47 43 and 46 (24)
Appendix 4. EMBASE search strategy
This search was first performed on 5 July 2012, and updated on 17 December 2013, 22 May 2014 and 12 March 2015.
1 exp prasterone/ (12189) 2 exp androgen/ or exp testosterone/ (137696) 3 dehydroepiandrosterone.tw. (10583) 4 DHEA.tw. (6136) 5 testosterone.tw. (69331) 6 prasterone$.tw. (111) 7 Androstenolone.tw. (48) 8 or/1‐7 (150418) 9 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (51835) 10 embryo$ transfer$.tw. (12448) 11 vitro fertili?ation.tw. (20598) 12 ivf‐et.tw. (2410) 13 icsi.tw. (9302) 14 intracytoplasmic sperm injection$.tw. (6212) 15 (blastocyst adj2 transfer$).tw. (1023) 16 (ivf or et).tw. (462469) 17 exp infertility therapy/ or exp artificial insemination/ or exp intrauterine insemination/ or exp ovulation induction/ (76617) 18 artificial insemination.tw. (4717) 19 intrauterine insemination.tw. (2357) 20 (infertil$ adj5 women).tw. (7934) 21 (subfertil$ adj5 women).tw. (522) 22 (infertil$ adj5 female$).tw. (2860) 23 (subfertil$ adj5 female$).tw. (170) 24 or/9‐23 (527601) 25 8 and 24 (4950) 26 Clinical Trial/ (890548) 27 Randomized Controlled Trial/ (361911) 28 exp randomization/ (64138) 29 Single Blind Procedure/ (18632) 30 Double Blind Procedure/ (119131) 31 Crossover Procedure/ (39190) 32 Placebo/ (230615) 33 Randomi?ed controlled trial$.tw. (97518) 34 Rct.tw. (13226) 35 random allocation.tw. (1316) 36 randomly allocated.tw. (20088) 37 allocated randomly.tw. (1944) 38 (allocated adj2 random).tw. (739) 39 Single blind$.tw. (14173) 40 Double blind$.tw. (142348) 41 ((treble or triple) adj blind$).tw. (343) 42 placebo$.tw. (198525) 43 prospective study/ (257444) 44 or/26‐43 (1396505) 45 case study/ (22990) 46 case report.tw. (257978) 47 abstract report/ or letter/ (895891) 48 or/45‐47 (1171324) 49 44 not 48 (1358996) 50 25 and 49 (849) 51 (201211$ or 201212$).em. (41825) 52 2013$.em. (1508153) 53 2013$.dp. (156466) 54 51 or 52 or 53 (1550697) 55 50 and 54 (110)
Appendix 5. PSYCINFO search strategy
This search was first performed on 5 July 2012, and updated on 17 December 2013, 22 May 2014 and 12 March 2015.
1 exp androgens/ or exp testosterone/ (5444) 2 dehydroepiandrosterone.tw. (757) 3 DHEA.tw. (553) 4 testosterone.tw. (7160) 5 prasterone$.tw. (2) 6 Androstenolone.tw. (1) 7 androgen$.tw. (4018) 8 or/1‐7 (9839) 9 exp reproductive technology/ (1260) 10 embryo$ transfer$.tw. (97) 11 vitro fertili?ation.tw. (520) 12 ivf‐et.tw. (17) 13 icsi.tw. (42) 14 intracytoplasmic sperm injection$.tw. (36) 15 (blastocyst adj2 transfer$).tw. (3) 16 (ivf or et).tw. (92948) 17 artificial insemination.tw. (219) 18 intrauterine insemination.tw. (14) 19 or/9‐18 (94144) 20 8 and 19 (284) 21 random.tw. (39303) 22 control.tw. (305573) 23 double‐blind.tw. (17481) 24 clinical trials/ (7170) 25 placebo/ (3640) 26 exp Treatment/ (562694) 27 or/21‐26 (859192) 28 20 and 27 (88) 29 limit 28 to yr="2012 ‐Current" (4)
Appendix 6. CINAHL database search
CINAHL search 22 May 2014. We updated this search on 12 March 2015.
| # | Query | Results |
| S43 | S28 AND S42 | 67 |
| S42 | S29 OR S30 or S31 or S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 | 891,110 |
| S41 | TX allocat* random* | 3905 |
| S40 | (MH "Quantitative Studies") | 12,016 |
| S39 | (MH "Placebos") | 8741 |
| S38 | TX placebo* | 31,574 |
| S37 | TX random* allocat* | 3905 |
| S36 | (MH "Random Assignment") | 37,244 |
| S35 | TX randomi* control* trial* | 72,875 |
| S34 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 715,853 |
| S33 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 105 |
| S32 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 0 |
| S31 | TX clinic* n1 trial* | 163,314 |
| S30 | PT Clinical trial | 75,963 |
| S29 | (MH "Clinical Trials+") | 174,859 |
| S28 | S9 AND S27 | 197 |
| S27 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 | 9322 |
| S26 | TX infertilit* | 6754 |
| S25 | TX subfertilit* | 333 |
| S24 | (MM "Infertility") | 3445 |
| S23 | TX ovarian hyperstimulation | 299 |
| S22 | TX superovulation | 19 |
| S21 | TX intrauterine insemination | 135 |
| S20 | TX IUI | 70 |
| S19 | TX artificial insemination | 435 |
| S18 | (MM "Ovulation Induction") | 207 |
| S17 | (MM "Insemination, Artificial") | 228 |
| S16 | TX embryo* N3 transfer* | 699 |
| S15 | TX ovar* N3 hyperstimulat* | 301 |
| S14 | TX ovari* N3 stimulat* | 223 |
| S13 | TX IVF or TX ICSI | 1134 |
| S12 | (MM "Fertilization in Vitro") | 1348 |
| S11 | TX vitro fertilization | 2672 |
| S10 | TX vitro fertilisation | 259 |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 | 6013 |
| S8 | TX prasterone | 18 |
| S7 | TX prasterone | 18 |
| S6 | TX testosterone | 4888 |
| S5 | TX dehydroepiandrosterone | 1036 |
| S4 | TX DHEA | 360 |
| S3 | (MM "Dehydroepiandrosterone") | 383 |
| S2 | (MM "Testosterone Replacement Therapy") | 35 |
| S1 | (MM "Androgens") | 767 |
Appendix 7. Trials registry search terms
"DHEA" AND "IVF", and "TESTOSTERONE" AND "IVF"
Appendix 8. Cochrane 'Risk of bias' assessment tool
| Domain | Support for judgement | Review authors' judgement |
| Selection bias | ||
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. |
| Performance bias | ||
|
Blinding of participants and personnel Assessments should be made for each main outcome (or class of outcomes) |
Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. |
| Detection bias | ||
|
Blinding of outcome assessment Assessments should be made for each main outcome (or class of outcomes) |
Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Detection bias due to knowledge of the allocated interventions by outcome assessors. |
| Attrition bias | ||
| Incomplete outcome data Assessments should be made for each main outcome (or class of outcomes) | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | Attrition bias due to amount, nature or handling of incomplete outcome data. |
| Reporting bias | ||
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Reporting bias due to selective outcome reporting. |
| Other bias | ||
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review's protocol, responses should be provided for each question/entry. |
Bias due to problems not covered elsewhere in the table. |
Data and analyses
Comparison 1. DHEA or testosterone versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth/ongoing pregnancy rate | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 DHEA | 8 | 878 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.25, 2.62] |
| 1.2 Testosterone | 4 | 345 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.60 [1.30, 5.20] |
| 2 Live birth rate, treatment‐naive women and having had previous ovarian stimulationive birth/ongoing pregnancy rate | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 DHEA ‐ treatment‐naive women | 2 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.53, 2.60] |
| 2.2 DHEA ‐ had previous ovarian stimulation | 6 | 754 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.34, 3.10] |
| 3 Live birth rate by length of DHEA administration | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 DHEA 6 weeks | 1 | 33 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.30, 34.64] |
| 3.2 DHEA 8 weeks | 2 | 161 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.30, 5.27] |
| 3.3 DHEA 12 to 15 weeks | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.87] |
| 3.4 DHEA 16 weeks | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.12, 8.13] |
| 3.5 DHEA 24 weeks | 1 | 406 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.23, 4.22] |
| 4 Live birth rate by length of T administration | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Up to 7 days | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.39, 8.27] |
| 4.2 14 to 20 days | 2 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.50, 8.88] |
| 4.3 21 to 28 days | 2 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.38, 7.23] |
| 5 Miscarriage rate | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 DHEA | 8 | 950 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.29, 1.17] |
| 5.2 Testosterone | 4 | 345 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.58, 7.13] |
| 6 Miscarriage rate, treatment‐naive women and having had previous ovarian stimulation | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 DHEA ‐ treatment‐naive women | 2 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.12, 1.63] |
| 6.2 DHEA ‐ had previous ovarian stimulation | 6 | 826 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.29, 1.50] |
| 7 Miscarriage rate by length of DHEA administration | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 DHEA 8 weeks | 2 | 161 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.84] |
| 7.2 DHEA 12 weeks | 2 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.20, 5.12] |
| 7.3 DHEA 12 to 16 weeks | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 78.04] |
| 7.4 DHEA 16 weeks | 2 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.13, 1.97] |
| 7.5 DHEA 24 weeks | 1 | 406 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.30, 6.06] |
| 8 Miscarriage rate by length of T administration | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Up to 7 days | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.74] |
| 8.2 14 to 20 days | 2 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.34, 16.49] |
| 8.3 21 to 28 days | 2 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.40, 10.68] |
| 9 Clinical pregnancy rate | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 DHEA | 12 | 1246 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.01, 1.76] |
| 9.2 Testosterone | 4 | 345 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.36, 4.68] |
| 10 Multiple pregnancy | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 DHEA | 5 | 267 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.13, 81.01] |
| 10.2 Testosterone | 3 | 292 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.48, 19.98] |
| 11 Adverse effects to the woman | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Increased acne | 2 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.40 [0.61, 47.63] |
1.1. Analysis.
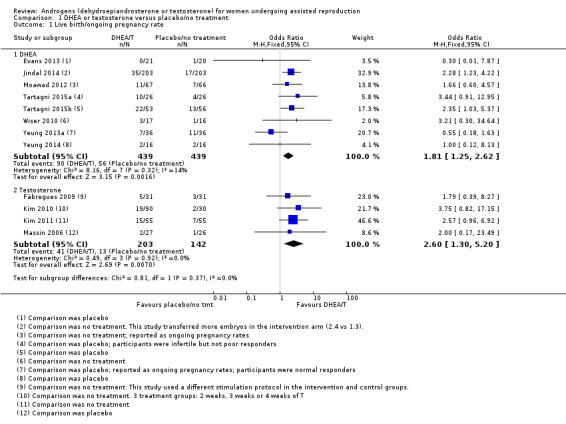
Comparison 1 DHEA or testosterone versus placebo/no treatment, Outcome 1 Live birth/ongoing pregnancy rate.
Comparison 2. Testosterone versus other active interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Testosterone vs estradiol | 1 | 46 | Risk Difference (M‐H, Fixed, 95% CI) | 0.10 [‐0.12, 0.32] |
| 1.2 Testosterone vs estradiol + OCP | 1 | 42 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.16, 0.31] |
| 2 Miscarriage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Testosterone vs estradiol | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.11, 4.64] |
| 2.2 Testosterone vs estradiol + OCP | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.9 [0.16, 22.72] |
| 3 Clinical pregnancy rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Testosterone vs estradiol | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.39, 5.07] |
| 3.2 Testosterone vs estradiol + OCP | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.45, 7.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Artini 2012.
| Methods | Patients were randomly divided in two groups by sealed, opaque, sequentially numbered envelopes. One group of 12 patients received DHEA supplementation for 3 months before and IVF cycle (study group). The other group of 12 patients did not receive any treatment (control group). All patients received combined oral contraceptives for a month before starting controlled ovarian hyperstimulation (COH). A flexible start antagonist protocol was used. | |
| Participants | This controlled, randomized study enrolled 24 patients, all diagnosed as poor responders based on the "Bologna criteria" for the definition of poor response, established during ESHRE consensus in 2010. All selected patients were aged between 31 and 42 years. There were no differences between treated and non‐treated group regarding mean age, BMI, duration of infertility, and baseline serum levels of FSH and DHEAS. |
|
| Interventions | DHEA supplementation orally, 25 mg three times daily. | |
| Outcomes | The primary outcome measures were hypoxic inducible factor 1 (HIF1), vascular endothelial growth factor (VEGF) concentrations in follicular fluid and the number of mature oocytes among the corresponding retrieved oocytes. Secondary outcome measures were number of retrieved oocytes, mature oocytes, fertilized oocytes, good quality embryos, transferred embryos and clinical pregnancies. Results were expressed as mean ± standard deviation (SD). Between‐group differences were evaluated by means of Student's t‐test. A P value of < 0.05 was considered statistically significant. |
|
| Notes | "The purpose of this study was to analyze the effect of DHEA supplementation firstly on follicular microenvironment, evaluating follicular fluid VEGF and HIF1 concentrations and, secondly, on IVF outcomes." Clinical pregnancy rates reported as means and SDs, clarification requested from trial authors but not received. We calculated clinical pregnancy rates from the data in the paper; the P value provided confirms our assumption was correct. We also asked for live birth, miscarriage and ongoing pregnancy data, and further information on their methods. No response at 5 June 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were divided in two groups by randomly choosing sealed, opaque, sequentially numbered envelopes." |
| Allocation concealment (selection bias) | Low risk | "sealed, opaque, sequentially numbered envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information given but it is clear participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Only one patient did not complete the cycle and was suspended because of insufficient response to COH, and she belonged to control group." Protocol requested. |
| Selective reporting (reporting bias) | Unclear risk | Protocol requested but not received. |
| Other bias | Low risk | No other source of bias detected. |
Divita 2003.
| Methods | Randomised double blind placebo‐controlled trial | |
| Participants | 31 infertile couples described as 'poor responders', age not stated | |
| Interventions | Participants were randomised to either a daily oral dose of 40 mg of micronised DHEA sulfate (DHEAS) or placebo, commencing at the same time as a GnRH agonist, in the mid‐luteal phase of the cycle prior to IVF. | |
| Outcomes | Pregnancy rates | |
| Notes | Conference abstract only 1 withdrawal/dropout from 31 couples randomised Unable to contact study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given about method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No details given about allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was stated to be placebo‐controlled but details lacking. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given. |
| Selective reporting (reporting bias) | Unclear risk | No information given. |
| Other bias | Unclear risk | Insufficient information to detect any other sources of bias. |
Evans 2013.
| Methods | A randomized double blinded placebo controlled single centre trial comparing the effect of treatment with DHEA on pregnancy outcome in women with resistant ovaries undergoing IVF and ICSI. 21 women received the active treatment, 20 women received placebo. | |
| Participants | Women 40 to 45 yrs with resistant ovaries, as defined by:
|
|
| Interventions | DHEA 75 mg oral capsule once daily for 4 months or placebo daily for 4 months prior to IVF | |
| Outcomes | Number of embryos Implantation rates Clinical pregnancy rate Live birth rate Side effects |
|
| Notes | Abstract only. Additional information received from study authors. A primary study objective was to determine the effect of treatment with DHEA on pregnancy outcome in women with resistant ovaries undergoing IVF and ICSI. "Trial discontinued because of staffing issues". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomized double blinded placebo controlled single centre trial. "The patient packs/study drugs were labelled with a unique patient identification number. When a patient had been found eligible for the study and completed all the baseline procedures, she was allocated a unique patient identification number in sequential, chronological order. The patient was then treated with the medication labelled with the same number. The treatment had already have been randomised at source by St Mary’s Pharmaceutical Unit. If a patient was withdrawn from the study after dispensing the drug, her identification number was not reallocated. The randomisation code for each patient was kept in the pharmacy Accountability Log for emergency use only. In the absence of any emergency, patient allocation details were kept coded and remained confidential until the trial completion. " |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blinded – to exclude patient / clinician effect" ‐ quote from study final report. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double blinded – to exclude patient / clinician effect" ‐ quote from study final report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes per protocol were reported. |
| Other bias | Low risk | No other source of bias detected. |
Fábregues 2009.
| Methods | "Randomised clinical trial" | |
| Participants | "62 infertile women with a background of a cancelled IVF treatment cycle due to poor follicular response" | |
| Interventions | Treatment group: "transdermal application of testosterone preceding standard gonadotrophin ovarian stimulation under GnRHa pituitary suppression" 2.5 mg/day nominal delivery rate; 20 μg/kg for 5 days Control group: ovarian stimulation with high‐dose gonadotrophin in association with a minidose GnRH agonist protocol" |
|
| Outcomes | Incidence of low‐responder patients, patients reaching ovum pickup, number of follicles, peak estradiol levels, clinical pregnancy rate | |
| Notes | Power calculation done | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were allocated to each group according to a computer generated randomization table". |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes for the randomization list were used" and authors confirm that envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware if they were in the treatment group. The trial authors believed that this was unlikely to introduce bias. The trial authors have confirmed that "study staff did not know the treatment allocations". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The study staff and outcome assessors do not know the treatment allocations". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or exclusions. |
| Selective reporting (reporting bias) | Low risk | No published protocol to refer to but refer 'proof of concept' trial Balasch 2006. Live birth rate reported. |
| Other bias | High risk | Patients randomised to the treatment group received a different stimulation protocol to those in the control group. |
Jindal 2014.
| Methods | 406 women were computer randomised to receive either DHEA or infertility treatment without DHEA supplementation. | |
| Participants | "406 infertile patients aged 32 ‐ 42 years" All patients were diagnosed as having diminished ovarian reserve or premature ovarian ageing |
|
| Interventions | 75 mg daily of oral, micronized DHEA for up to 6 months prior to entry into a repeat IVF‐ICSI cycle. IVF cycles undertaken were a mix of agonist and antagonist protocols (day 2/3 FSH of < 12 mL U/mL). | |
| Outcomes | The primary outcome was clinical pregnancy rate. CPR, LBR and miscarriage were reported. |
|
| Notes | Oral presentation at ESHRE 2014 with published abstract In response to a question, PI stated that assessors and participants were blinded. The abstract says controls "did not use DHEA", no mention of placebo there or in conference slides. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised. |
| Allocation concealment (selection bias) | Unclear risk | Information requested but not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "control group had infertility but did not receive DHEA". In response to a question, PI stated that assessors and participants were blinded, cannot be correct as controls had no treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess whether all women completed the study. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported. |
| Other bias | Unclear risk | Insufficient information to detect any other sources of bias. |
Kara 2014.
| Methods | Patients were allocated randomly to groups 1 and 2 on the day of the study. 104 in the study group and 104 in the control group. | |
| Participants | The study included 208 consecutive patients undergoing IVF‐ICSI because of infertility and diminished ovarian reserve. Patients whose serum AMH level < 1 ng/mL or serum FSH value > 15 iu/L and antral follicle count < 4 on the second day of the menstruation were accepted as having diminished ovarian reserve. | |
| Interventions | DHEA 25 mg three times daily was administered throughout 12 weeks in study group (Group 1). In control group (group 2) only IVF‐ICSI was performed. All patients underwent microdose flare protocol. |
|
| Outcomes | Retrieved oocyte number Fertilization rate Clinical pregnancy rate |
|
| Notes | "The aim of this study was to evaluate the efficacy of Dehydroepiandrosterone (DHEA) on IVF‐ICSI outcome of the poor responder patients. Retrieved oocyte number and fertilization rate were slightly higher in study group but the pregnancy rate was better in control group. The differences were not statistically significant." "Because the institution is private and specialized for the IVF. We have limited data about the patients’ antenatal care and the pregnancy outcome. Thus we don't have any further data we can share." ‐ personal communication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐assisted randomization was used according to the instructions at www.randomization.com. Women were allocated at random to the study group or the control group on the day of the study." |
| Allocation concealment (selection bias) | Unclear risk | "We did not use the sealed opaque envelopes." ‐ personal communication. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patients were not blind to treatment but the doctors managing the fertility treatment were blind." ‐ personal communication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients were not blind to treatment but the doctors managing the fertility treatment were blind." ‐ personal communication. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusions were made at the end of DHEA administration, so that not all randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | Details not supplied but has reported outcomes relevant to this review. |
| Other bias | Unclear risk | Insufficient information to detect any other sources of bias. |
Kim 2010.
| Methods | Prospective randomised study | |
| Participants | 120 low‐responders | |
| Interventions | 12.5 mg testosterone transdermal gel administered daily for 2 weeks, 3 weeks or 4 weeks in the cycle preceding COS using GnRH antagonist multiple‐dose protocol | |
| Outcomes | Oocytes retrieved, mature oocytes and fertilised oocytes (IVF outcomes). Significantly higher numbers of oocytes retrieved, mature oocytes and fertilised oocytes after 3 and 4 weeks. Per email reported live birth rate, clinical pregnancy rate and miscarriage rate. |
|
| Notes | Conference abstract only. Further information supplied by the corresponding authors (email 8 May 2013). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated into the treatment groups (2 weeks, 3 weeks or 4 weeks TTG pretreatment groups) and no treatment group by the use of sealed envelopes and a computer‐generated list." |
| Allocation concealment (selection bias) | Unclear risk | "The sequence of allocation to the four groups was provided to the investigating physicians, and randomization was performed as planned according to the randomization list order." "After the sealed envelopes for randomization were opened, it was possible to see what the allocation was." Not stated whether envelopes were opaque and serially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study was open‐label study, and therefore the study staff and outcome assessors were not blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The study was open‐label study, and therefore the study staff and outcome assessors were not blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women completed the study. |
| Selective reporting (reporting bias) | Low risk | Details not supplied but has reported outcomes relevant to this review. |
| Other bias | Unclear risk | Insufficient information to detect any other sources of bias. |
Kim 2011.
| Methods | Prospective, randomised controlled study | |
| Participants | 110 low responders (who had failed to produce > 3 oocytes in a previous IVF cycle using high dose > 2500iu FSH stimulation), who underwent 110 IVF/ICSI cycles between March 2005 and January 2009. All participants were given oestradiol and progesterone pretreatment for 21 days prior to COH using an antagonist protocol. | |
| Interventions | Group A (N = 55): once daily application of 12.5 mg TTG (Testo gel 1%) started from the 6th day of oestrogen‐progesterone pretreatment and continued for 21 days Group B (N = 55): no gel treatment |
|
| Outcomes | Primary outcome was the number of mature oocytes retrieved. Secondary outcomes included live birth rate, clinical pregnancy rate, miscarriage rate, twin pregnancy rate. | |
| Notes | One to four embryos transferred in both groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated …by the use of sealed envelopes and a computer generated list". |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomly allocated …by the use of sealed envelopes and a computer generated list". Comment: the opacity of the envelopes was unclear, no info regarding numbering, changed to 'unclear' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded as there was no placebo treatment therefore those applying gel knew they were in the treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinicians were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | As far as it is known, ITT analysis was applied. |
| Selective reporting (reporting bias) | Low risk | Unable to find a published protocol but has reported outcomes relevant to this review. |
| Other bias | Unclear risk | insufficient information to detect any other sources of bias. |
Marzal 2014.
| Methods | Randomised, open label Priming with T, Estradiol (E) or a combination of E and OCP (oral contraceptive pill) prior to ICSI |
|
| Participants | Women 18 years to 38 years; confirmed low responder patients Phase I: (Non‐randomized) Identification of low responder patients. Potential low responder patients underwent a standardized ovarian hyperstimulation protocol. Phase II: (Randomized) Those patients confirmed as low responders in phase 1 of the trial were offered the opportunity to enter the interventional part of the study. |
|
| Interventions | In Phase II, women were randomized to three different treatment groups: estradiol, testosterone or combined progestagens and estrogens prior to the IVF‐ICSI cycle. The previous cycle (phase I) was used as a self‐control for each patient. 3 arms ‐ T from day 24 of previous cycle until day 2 of ICSI cycle (6 days)(22 women), E (24 women) or E + OCP (20 women) Testosterone was administered as a testosterone patch, 20 µg/kg/day. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
NCT01310647 Data advised by PI ‐ per email 2/6/14 "99 women did the first COH, 84 were poor responders, but only 66 were randomized to the second COH + priming." "There were 3 patients who didn't receive the priming: 1 in the estradiol group and 2 in the estradiol+OCP group." "Effectively this was an open trial. Only analysts were blinded (interventions were codified as 1,2 and 3." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A pure allocation method was used. A random sequence was generated using 'Ramdom.org'." |
| Allocation concealment (selection bias) | Unclear risk | Details not supplied. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label, no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "all women randomised were included in the analysis". |
| Selective reporting (reporting bias) | Low risk | Details not supplied but has reported outcomes relevant to this review. |
| Other bias | Unclear risk | Insufficient information to detect any other sources of bias. |
Massin 2006.
| Methods | "prospective, randomized, double‐blind, placebo‐controlled study" Design: "paired comparison of the ovarian parameters recorded in two consecutive cycles, each woman being used as her own control". First cycle defined as control cycle, second as the treatment cycle. Both cycles used comparable stimulation in a GnRH agonist protocol. |
|
| Participants | 53 women aged < 42 with both poor ovarian response in a previous IVF or ICSI attempt and evidence of decreased ovarian reserve ‐ defined as plasma estradiol (E2) value < 1200 pg/mL at HCG day and retrieved oocytes ≤ 5, and evidence of decreased ovarian reserve by day 3 FSH, E2 or Inhibin B outside of the normal range (FSH > 12 IU/L, E2 > 70 pg/mL and inhibin B < 45 pg/mL). | |
| Interventions | Transdermal testosterone gel (once‐daily 1 g of gel; 10 mg of testosterone) vs placebo gel; applied for 15 to 20 days preceding the second stimulation for IVF or ICSI. | |
| Outcomes | Number of oocytes retrieved, implantation rate, clinical pregnancy rate, delivery rate "multiple pregnancy : not reported". "These data were not properly recorded and are not available." "1 spontaneous pregnancy in the testogel group discovered at desensitization with a miscarriage...This spontaneous pregnancy was observed in the testogel treated cycle at the time of desensitization, before ovarian stimulation. It also ended in early miscarriage. This patient was not included in the group of 24 patients treated with Testogel." "testogel 4 clinical pregnancies : 2 miscarriages and 2 live birth placebo 2 clinical pregnancies (+2 biochemical pregnancies) : 1 miscarriage and 1 live birth" "There were actually 2 pregnancies in the control group but 1 of them was the result of IUI because ovarian retrieval was cancelled. It was not included in the final analysis. This pregnancy ended in early miscarriage." |
|
| Notes | Power calculations carried out. Further information supplied by corresponding author (email of 17 April 2013). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "allocation sequence was generated by a random permutation table (blocks of four)". |
| Allocation concealment (selection bias) | Unclear risk | "women were enrolled by only one physician who assigned consecutive numbers to women, by order of inclusion". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Since placebo gel was used, it was impossible for participants to know if they were receiving the treatment. Corresponding author confirmed that the physician assigning the allocation numbers to the women was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "ultrasonography was performed...by the same physician who was not informed of patient's treatment allocation". Q = Were the outcome assessors blinded to the treatment allocations? A = "Yes treatment allocation unblinded after the last patient out, when the statistical analysis was performed". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis was not used; 24/27 women randomised to treatment group and 25/26 women in control group were analysed. |
| Selective reporting (reporting bias) | Low risk | Could not find the protocol of the trial on clinicaltrials.gov details not supplied but has reported outcomes relevant to this review. |
| Other bias | Low risk | No other source of bias detected. |
Moawad 2012.
| Methods | Prospective study including 133 couples attending the IVF programme between July 2008 and April 2012. The cases were randomised into 2 groups; (1) study group (DHEA group), (2) control group; in which the patient started COH without DHEA priming. All patients were treated according to the standard short stimulation protocol with GnRH agonist The randomization was according to a computer‐generated list. Cases were sequentially allocated to either the study or the control group in strict compliance with the sequence of cases and the order of allocation groups. Computer‐generated randomization was by the nurse coordinator without interference from the clinicians. |
|
| Participants | Patients younger than 40 years old with a prior poor response to ovarian stimulation in IVF were selected to undergo an IVF cycle. Patients who received DHEA at any time before enrolment or patient with AMH levels more than 1.7 lg/L were excluded. |
|
| Interventions | Study group: 25 mg of DHEA orally, three times a day, for at least 12 weeks before starting ovulation induction. The DHEA was dispensed by a single pharmacy to all study group participants. | |
| Outcomes | The primary outcome measures were peak estradiol levels, number of retrieved oocytes and number of embryos reserved for transfer. Secondary outcome measures were pregnancy and clinical pregnancy rates. "It should be noted that two patients in the study group conceived spontaneously 45 days after DHEA exposure, before starting IVF treatment and was included among the study group pregnancies." "In the current study, pregnancy rate (per embryo transfer) was higher in the DHEA group (24.1%) compared to the control group (21.3%) with no significant difference. However, if we calculate pregnancy rate per cycle, the DHEA group had a significantly higher pregnancy rate (20.9%) compared to (15.2%) the control group (P value = 0.048, significant)." Also, clinical pregnancy and ongoing pregnancy (per embryo transfer) were significantly higher in the DHEA group (20.7% and 19.0%) compared to the control group (17.0% and 14.9%), respectively (P value = 0. 048 and 0.041, significant). "The medication was well tolerated by all patients. No patient dropped out of treatment because of side effects attributed to DHEA use." |
|
| Notes | Intention was to transfer two or three embryos per participant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was done according to a computer‐generated list. Cases were sequentially allocated to either the study or the control group in strict compliance with the sequence of cases and the order of allocation groups. This computer‐generated randomization was done by the nurse coordinator without interference from the clinicians." |
| Allocation concealment (selection bias) | Unclear risk | "The randomization was done according to a computer‐generated list. Cases were sequentially allocated to either the study or the control group in strict compliance with the sequence of cases and the order of allocation groups. This computer‐generated randomization was done by the nurse coordinator without interference from the clinicians." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group unblinded as received no treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | Details not stated but has reported outcomes relevant to this review. |
| Other bias | Unclear risk | Insufficient information to detect any other sources of bias. |
Tartagni 2015a.
| Methods | Double‐blind, randomized, placebo‐controlled study | |
| Participants | 52 infertile patients | |
| Interventions | All women received the long protocol IVF. Patients in Group 1, received 75 mg of DHEA once a day, 8 weeks before starting the IVF cycle and during treatment; control group (Group 2) received placebo. | |
| Outcomes | The primary endpoints were pregnancy, live birth and miscarriage rates. Secondary endpoint was standard IVF parameters such us stimulation duration (hCG day), E2 on HCG‐day, endometrial thickness, number of retrieved oocytes, metaphase II oocytes, embryos transferred and score of leading embryos transferred. |
|
| Notes | Women described as infertile but not poor responders. Trial authors confirm: allocation list was managed by an independent subject of the institution not directly involved in the trial (nurse). Study personnel and outcome assessors were blinded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a computer‐generated randomization sequence". |
| Allocation concealment (selection bias) | Low risk | "Allocation list was managed by an independent subject of the institution not directly involved in the trial (nurse)". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial authors confirm that participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial authors confirm that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women completed the trial. |
| Selective reporting (reporting bias) | Low risk | Authors appear to have reported all planned outcomes. |
| Other bias | Low risk | Nothing detected. |
Tartagni 2015b.
| Methods | Computer‐generated randomization sequence, paper silent on allocation concealment. | |
| Participants | 109 patients with a diagnosis of infertility and undergoing their first IVF cycle were consecutively recruited between January 2010 and October 2012 among those coded in the anonymous research database of our academically affiliated private infertility centre. All patients enrolled had unsuccessfully sought to become pregnant for more than 3 years and had failed at least 3 intrauterine inseminations (IUI). | |
| Interventions | Group 1 (N = 53) patients DHEA (75 mg/die, orally) 8 weeks before starting the cycle of ovulation induction. Group 2 (N = 56) received placebo during the same period. Both DHEA and placebo were administered throughout the whole period of ovarian stimulation up to β‐HCG test. DHEA dispensed in this study was obtained by a single pharmacy. |
|
| Outcomes | Primary live birth, clinical pregnancy, miscarriage, Secondary end‐points were standard IVF parameters, such as stimulation duration (days of rhFSH treatment), E2 levels on hCG‐day, endometrial thickness, number of retrieved oocytes, number of metaphase II oocytes, number of embryos transferred and score of leading embryos transferred. |
|
| Notes | Supported by a research grant award (PRIN 2010YK7Z5K_008) to M.Montagnani from the Italian Ministry of University and Research (MIUR). Trial authors confirm per email: allocation list was managed by an independent subject of the institution not directly involved in the trial (nurse). Study personnel and outcome assessors were blinded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "On the basis of a computer‐generated randomization sequence". |
| Allocation concealment (selection bias) | Low risk | "allocation list was managed by an independent subject of the institution not directly involved in the trial (nurse)". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial authors confirm that participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial authors confirm that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients in both groups completed the study." |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | Nothing detected. |
Wiser 2010.
| Methods | "Randomised, prospective, controlled study" | |
| Participants | 33 women with diminished ovarian reserve who underwent 51 cycles (ie the study protocol was a maximum of 2 treatment cycles). A poor response in a previous IVF cycle was defined as retrieval of fewer than five oocytes, poor‐quality embryos, or cycle cancellation due to poor response to ovarian stimulation (Frattarelli et al., 2008), whenever the gonadotrophin starting dose for induction of ovulation was at least 300 IU/day. Exclusions: over age of 42, or having received DHEA at any time before treatment |
|
| Interventions | 75 mg DHEA (oral) once a day for at least six weeks before starting first cycle of COH. Women who did not conceive and continued to the second cycle (9 in each group) took DHEA for at least 16 to 18 weeks before the second cycle. All women were treated with long GnRH agonist protocol IVF. |
|
| Outcomes | Clinical pregnancy rate, live birth rate; reported after both cycles. Only first cycle data used in this review. | |
| Notes | NCT01145144 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the randomization was performed using computer generated random numbers". |
| Allocation concealment (selection bias) | Unclear risk | "each patient chose a sealed envelope containing the randomised assignment to either the study or control group". Comment: The opacity of the envelopes was unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants knew if they were in the treatment group as they had to take the DHEA. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All randomised women were analysed however drop outs after cycle 1 were high: 25% in the intervention group and 31% in the control group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on were recorded in the protocol. |
| Other bias | Unclear risk | Insufficient information to detect any other sources of bias. |
Yeung 2013a.
| Methods | Randomized double‐blinded placebo‐controlled study. Normal responders were randomized into DHEA (N = 36) and placebo groups (N = 36) according to a computer generated randomization list. Monthly ovarian reserve markers (AFC, AMH, FSH) were documented.In the third month. Standard dose HMG stimulation in a long agonist protocol was undertaken and outcomes were compared. |
|
| Participants | Normal responders (defined as AFC 5‐15) were recruited from a university‐affiliated reproductive unit between 10/2010 and 5/2012. | |
| Interventions | DHEA 25 mg tds or placebo were started 12 weeks prior to IVF. | |
| Outcomes | AFC, FSH, AMH, duration and dose of gonadotrophin use, number of follicles number of top quality embryos; clinical pregnancy rates and ongoing pregnancy rates | |
| Notes | To assess whether DHEA supplementation in women with normal responders improve ovarian reserve markers, ovarian response to standard dose gonadotrophins or IVF outcomes. Similar clinical pregnancy rates (27.8 vs 41.1%, P = 0.347) and ongoing pregnancy rates (19.4 vs 29.4%, P = 0.521) were observed in DHEA and placebo groups. Miscarriage not reported. Study protocol supplied by authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized into DHEA (n=36) and placebo groups (n=36) according to a computer generated randomization list". |
| Allocation concealment (selection bias) | Low risk | "Randomized double‐blinded placebo‐controlled study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Randomized double‐blinded placebo‐controlled study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Randomized double‐blinded placebo‐controlled study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported per study protocol. |
| Other bias | Low risk | No other source of bias detected. |
Yeung 2014.
| Methods | Subjects were recruited between 10/2010 and 8/2012 in a university‐affiliated reproductive unit and randomized into DHEA (N = 16) and placebo groups (N = 16) according to a computer generated randomization list. DHEA or placebo were started 12 weeks prior to IVF. Monthly ovarian reserve markers (AFC, AMH, FSH), ovarian response to low dose ovarian stimulation in the third month and also subsequent IVF outcomes were compared. | |
| Participants | Women attending the Subfertility Clinic at the Department of Obstetrics and Gynaecology, University of Hong Kong, who were indicated for IVF treatment. Inclusion criteria: (1) age ≤40 years, (2) subfertility > 1 year, and (3) expected poor ovarian response defined as AFC < 5. Exclusion criteria: (1) had a history of ovarian cystectomy or oophorectomy, (2) had received cytotoxic chemotherapy, (3) had received pelvic irradiation, or (4) had a history of taking testosterone or DHEA supplementation. |
|
| Interventions | Baseline evaluations on day 2 of the menstrual cycle 12 weeks before scheduled IVF treatment. Pretreatment with DHEA 25 mg three times daily or placebo for 12 weeks before scheduled IVF treatment. Ovarian stimulation: at week 8, low‐dose gonadotropin stimulation using 75 IU of human menopausal gonadotropin (hMG, Menogon; Ferring Pharmaceuticals) was given on days 2 to 8 as a standardized test for ovarian response. Ovarian response was assessed on day 10 by the number of follicle(s) > 10 mm and serum E2 levels. IVF treatment: At week 12, the women were treated with ovarian stimulation under the fixed antagonist protocol. |
|
| Outcomes | AFC; serum E2; gonadotrophins used; Number of oocytes retrieved; number of fertilized embryos; transferable embryos; ongoing pregnancy rate (OPR); cycle cancellation; clinical pregnancy rate; live birth rate "One patient from the DHEA group discontinued the intervention before week 4 complaining of increased acne." |
|
| Notes | To assess whether DHEA supplementation in poor responders can improve ovarian reserve markers, ovarian response to stimulation with a standard dose of gonadotrophin or outcomes in IVF treatment. Supported by: Committee on Research and Conference Grants, University of Hong Kong. Study protocol supplied by authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were randomized in a 1:1 ratio according to a computer‐generated randomization list generated by a research nurse not involved in the subjects' clinical management." |
| Allocation concealment (selection bias) | Low risk | "and were allocated in sealed, opaque, sequentially numbered envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The physicians and research nurses involved and the study participants were all blinded to the assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The physicians and research nurses involved and the study participants were all blinded to the assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | The authors have reported all proposed outcomes. |
| Other bias | Low risk | No other source of bias detected. |
Zhang 2014.
| Methods | Women were recruited between March 2013 and May 2014 in a university‐affiliated reproductive unit and randomized to DHEA 25 mg 3 times per day for three consecutive menstrual cycles prior to IVF cycles (N = 52), or to direct entry to IVF cycles (N = 53) according to a computer generated randomization list. All women had one IVF cycle (per author email 17/3/15). | |
| Participants | 105 women with diminished ovarian reserve. DOR was defined as: 1) an elevated day 3 FSH level ≥ 10 mIU/mL or FSH/LH > 3; 2) antral follicle count less than five; 3) a previous poor ovarian response: retrieval of fewer than five oocytes or cycle cancellation due to poor response to ovarian stimulation. A diagnosis with DOR was reached if they fulfilled any of the above three. Exclusion criteria included a history of ovarian cystectomy or oophorectomy; a diagnosis of endometriosis; or a history of DHEA supplementation or hormonal replacement therapy. |
|
| Interventions | Women in the DHEA group received DHEA 25 mg three times per day for three consecutive menstrual cycles prior to IVF cycles. Women in the control group entered IVF cycles directly. All women had 225 IU HMG combined with clomiphene citrate 100mg daily, and 10,000 IU of HCG when at least one mature follicle of ≥ 18 mm in mean diameter was seen on ultrasound; oocyte retrieval followed 36 hours later. |
|
| Outcomes | The primary outcome measures were follicular fluid BMP‐ 15, GDF‐9 and serum AMH, FSH, E² levels. Secondary outcome measures were the number of oocytes retrieved, MII oocytes, and embryos transferred; the accumulated score of embryos. Miscarriage reported per email 17/3/15: "Of the eight patients conceived in the DHEA group, two miscarried, with a miscarriage rate of 4.76%. In the control, two patients miscarried, with a miscarriage rate of 3.78%." Adverse events reported per email 17/3/15: "One patient complained of dizziness and three patients complained of acne were all in the DHEA group." |
|
| Notes | The paper reports 'pregnancy rates' but this was not defined. Authors were emailed on 12 March 2015, and confirm that this is clinical pregnancy. Funding sources (2) stated. Trial registration: ChiCTR‐TRC‐14005002 Adverse events reported per email 17/3/15: "One patient complained of dizziness and three patients complained of acne were all in the DHEA group." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "According to a computer‐generated randomization list by a nurse". |
| Allocation concealment (selection bias) | High risk | "This was not a blinded study. Both the patients and the doctors knew which group the patients were in." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This was not a blinded study. Both the patients and the doctors knew which group the patients were in." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "This was not a blinded study. Both the patients and the doctors knew which group the patients were in." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants accounted for, but 9 women withdrew for personal reasons, all from the treatment group. Authors report: "During the pretreatment of DHEA, 5 of them became bored and asked for IVF treatment. 2 of them went to other Reproductive Centers. And the other two did not give us any reason." |
| Selective reporting (reporting bias) | Unclear risk | Authors confirm that participants were not followed to live birth, only to clinical pregnancy. And per email reported miscarriage rates also. |
| Other bias | Unclear risk | Nothing detected. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balasch 2006 | Non‐randomised self‐controlled study. |
| Barad 2006 | Case‐control study. |
| Barad 2007 | Case‐control study. |
| Barad 2008b | Study terminated for failure to recruit sufficient participants. NCT00419913; insufficient information to assess eligibility. |
| Casson 2000 | Case series. |
| de los Santos 2013 | Case‐control study. |
| Fusi 2013 | Cohort study. |
| Fábregues 2011 | Dr Fábregues reported on 21 May 2014 that funding problems prevented completion of the study, and it has been stopped. NCT01291212. |
| Gleicher 2013 | Cohort study. |
| Hyman 2013 | Not a RCT. |
| Monterde 2013 | No randomised control group ‐ each treatment group its own control in previous cycle. |
| Motta 2006 | Not a RCT. |
| Singh 2013 | Not a RCT. |
| Sipe 2010 | Participants not women trying to conceive. |
| Sönmezer 2009 | Not a RCT. |
| Yeung 2013b | Participants not undergoing assisted reproduction. |
Characteristics of ongoing studies [ordered by study ID]
Barad 2008a.
| Trial name or title | Study of Oral Dehydroepiandrosterone (DHEA) to Treat Previously Unexplained Infertility |
| Methods | Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: factorial assignment Masking: double blind (subject, caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Women aged 21 to 37 years with > 1 year infertility |
| Interventions | Dietary supplement: DHEA (25 mg oral, 3 times per day) vs. placebo comparator |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | March 2008 |
| Contact information | David Barad, Centre for Human Reproduction, New York dbarad@theCHR.com |
| Notes | NCT00650754, (a study by the same investigator was terminated in 2008: NCT00419913) recruiting till March 2015 |
Barad 2012.
| Trial name or title | A Randomized Double Blind Control Trial of Transdermal Testosterone Supplementation vs Placebo on Follicular Development and Atresia, Oocyte and Embryo Quality Among Women With Diminished Ovarian Reserve Undergoing in Vitro Fertilization |
| Methods | Randomized; parallel assignment; double blind (subject, outcomes assessor) |
| Participants | Women with evidence of diminished ovarian reserve 38 to 44 years old planning to undergo ovulation induction for IVF who are willing to sign an informed consent. |
| Interventions |
Intervention
Drug: Testosterone cream (0.5 mg per g) 2g per day applied transdermally to the left wrist to deliver 1 mg daily dose with estimated absorption of 100 µg per day testosterone + DHEA Control DHEA + Placebo cream |
| Outcomes | Primary outcomes: Clinical and Ongoing Pregnancy (time frame: 8 weeks post treatment initiation) (Clinical pregnancy is defined as the presence of a viable gestational sac visible in the uterus 4 weeks after embryo transfer. Clinical ongoing pregnancy is defined as intrauterine pregnancy with evidence of an active fetal heart at 6 weeks after embryo transfer). Secondary outcomes: Measures of atresia (time frame: 8 weeks after intervention initiation)
Oocytes number (time frame: 8 weeks after initiation of intervention). The number of oocytes retrieved at oocyte retrieval for IVF will be compared between the treatment group and placebo. |
| Starting date | July 2012 End date: December 2014 |
| Contact information | Contact: Jolanta Tapper 212‐994‐4400 jtapper@theCHR.com Principal Investigator: David H Barad, MD MS Principal Investigator: Norbert Gleicher, MD |
| Notes |
NCT01662466 Still recruiting at 21 May 2014 |
Jayaprakasan 2012.
| Trial name or title | Efficacy of Dehydroepiandrosterone to Overcome the Effect of Ovarian Aging ‐ A Pilot Double Blinded Randomised Controlled Trial |
| Methods | Randomised, double‐blind, intervention study |
| Participants | Inclusion:
Exclusion criteria:
|
| Interventions | Drug: DHEA (St Mary's Pharmaceutical Unit Cardiff and Vale) DHEA 75 mg capsule. 1 capsule taken once daily for at least 12 weeks prior commencing ovarian stimulation protocol. Stimulation protocol standard long down‐regulation protocol, using human menopausal gonadotropin (HMG). Placebo: matched placebo containing no active ingredient. 1 capsule taken once daily for at least 12 weeks prior commencing ovarian stimulation protocol. Stimulation protocol standard long down‐regulation protocol, using human menopausal gonadotropin (HMG). |
| Outcomes | Primary outcomes:
Secondary outcomes: Oocyte quality (molecular markers) (time frame: the sample is collected at 14 to 16 weeks after recruitment and assessment is performed at one year.) Oocyte quality is assessed by expression of cumulus cell molecular markers of oocyte competence and also by assessing energy consumption (pyruvate, lactate and glucose utilization) from the media by the oocytes and embryos (nutritional finger printing). Aneuploidy rates in the immature oocytes and unfertilized oocytes using microarray technology (time frame: the sample is collected at 14 to 16 weeks after recruitment and assessment is performed at one year.) |
| Starting date | June 2012 |
| Contact information | Kannamannadiar Jayaprakasan, K.Jayaprakasan@nottingham.ac.uk |
| Notes |
NCT01572025 recruiting ongoing at 22 May 2014, preliminary results expected around August 2014. Preliminary information from study author that one woman dropped out of the study because the treatment made her feel sick. |
Kolibianakis 2014.
| Trial name or title | Transdermal Testosterone Pretreatment in Poor Responders Undergoing IVF |
| Methods | Intervention study; single blind (outcomes assessor) |
| Participants | 50 women; Advanced maternal age (≥ 40 years) |
| Interventions | 10 mg of testosterone gel applied on the external side of the thigh for 21 days starting from the first day of menstruation prior to initiation of ovarian stimulation with rFSH for IVF/ICSI vs no treatment |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | October 2013 Estimated primary completion date: October 2014 (final data collection date for primary outcome measure) |
| Contact information | Efstratios M Kolibianakis, MD, MSc, PhD stratis.kolibianakis@gmail.com Julia K Bosdou, MD, MSc juliabosdou@gmail.com |
| Notes | Still recruiting at May 2014 |
Tsafrir 2007.
| Trial name or title | DHEA Supplementation for Low Ovarian Response IVF Patients |
| Methods | Randomized; parallel assignment; open label |
| Participants | 18 to 43 years undergoing IVF |
| Interventions | DHEA 75 mg daily vs no treatment |
| Outcomes | Not stated |
| Starting date | Jan 2008 |
| Contact information | Avi Tsafrir, avits@szmc.org.il PI Ofer Gonen, ofer.gonen@clalit.org.il |
| Notes |
NCT00549081 Nothing per clinicaltrials.gov since 2007/8, no response to email 22 May 2014 |
Viardot‐Foucault 2012.
| Trial name or title | Study of Dehydroepiandrosterone Treatment for Poor Responders in In Vitro Fertilization Patients |
| Methods | Randomised, parallel assignment; open label |
| Participants | Women aged 21 to 42 years, poor responders |
| Interventions | DHEA 25 mg caps 3 x day vs no treatment |
| Outcomes | Primary:
Secondary:
|
| Starting date | February 2012 |
| Contact information | Veronique Viardot‐Foucault c/‐ hospital kkivf@kkh.com.sg |
| Notes |
NCT01535872 Ongoing but not recruiting, according to clinicaltrials.gov. No response to email 22 May 2014. |
Vlahos 2014.
| Trial name or title | Prospective Randomized Trial on the Effect of DHEA Administration in Women With Poor Ovarian Reserve Undergoing Controlled Ovarian Stimulation for IVF. Impact on Stimulation Characteristics and Pregnancy Outcome. |
| Methods | Randomized, parallel assignment, single blind (outcomes assessor) |
| Participants | 20 to 50 years |
| Interventions | DHEA 25 mg 3 x day orally for 12 weeks vs no treatment |
| Outcomes | Primary: CPR (time frame: at 12 weeks after DHEA administration and at 18 months). Secondary: changes in ovarian reserve indexes (time frame: 6 months). |
| Starting date | April 2014 |
| Contact information | Nikos Vlahos nikosvlahos@med.uoa.gr Olga Triantafillidou triantafyllidouolga@yahoo.com |
| Notes | NCT02099916 |
Differences between protocol and review
Live birth and ongoing pregnancy rates have been combined in reporting our primary outcome.
We have decided not to pool the results for DHEA and testosterone, on advice from referees and clinicians.
We have added some details to the secondary adverse effects outcomes. This includes specifying adverse events resulting from the treatment administered. We have changed the analysis method from Peto ORs to Mantel‐Haenszel ORs. In addition we reduced the number of comparisons proposed.
One included study reported multiple interventions; we have added provision for this in the Data extraction and management section of the Methods and referenced Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Chapter 16.5.4).
We added the following subgroup analyses:
Duration of treatment.
Previous ovarian stimulation status.
Contributions of authors
Helen Nagels developed the draft protocol and was the lead author in writing the protocol and full review.
Josephine Rishworth helped with all aspects of writing the review and was involved in review design, searching, data extraction and summary.
Charalampos Siristatidis and Ben Kroon reviewed the draft versions of the protocol, Nagels 2012, and full review, and as fertility specialists provided clinical expertise.
Sources of support
Internal sources
Menstrual Disorders and Subfertility Group, University of Auckland, Auckland, New Zealand.
External sources
None, Other.
Declarations of interest
None known
New
References
References to studies included in this review
Artini 2012 {published data only}
- Artini PG, Simi G, Ruggiero M, Pinelli S, Berardino OM, Papini F, et al. DHEA supplementation improves follicular microenvironment in poor responder patients. Gynecological Endocrinology 2012;28(9):669–73. [DOI] [PubMed] [Google Scholar]
Divita 2003 {published data only}
- Divita AE, Kanzepolsky LS, Notrica JA, Neuspiller FD, Polak de Fried E. Does dehidroepiandrosterone sulfate (Dheas) co‐treatment improve art outcome? A prospective, randomized, double‐blind placebo‐controlled trial. Fertility and Sterility 2003;80 Suppl 3:S111‐2. [Google Scholar]
Evans 2013 {published data only}
- Evans J. A randomised, double blinded, placebo controlled, single centre trial comparing dehydroepiandrosterone (DHEA) and placebo in the treatment of women with resistant ovaries prior to in vitro fertilisation (IVF) and intra cytoplasmic sperm injection (ICSI). RCOG World Congress; 2013 June 24‐26; Liverpool, UK. BJOG. 2013; Vol. EP2.93:225.
Fábregues 2009 {published data only}
- Fábregues F, Peñarrubia J, Creus M, Manau D, Casals G, Carmona F, et al. Transdermal testosterone may improve ovarian response to gonadotrophins in low‐responder IVF patients: a randomized, clinical trial. Human Reproduction 2009;24(2):349‐59. [DOI] [PubMed] [Google Scholar]
Jindal 2014 {published and unpublished data}
- Jindal A, Singh R. A prospective randomised controlled study on the role of dehydroepiandrosterone (DHEA) on improving ovarian response in known poor responders in previous failed IVF‐ICSI cycles. Human Reproduction. 2014; Vol. 29:Supp 1, i14.
Kara 2014 {published data only}
- Kara M, Aydin T, Aran T, Turktekin N, Ozdemir B. Does dehydroepiandrosterone supplementation really affect IVF‐ICSI outcome in women with poor ovarian reserve?. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;173:63‐5. [DOI] [PubMed] [Google Scholar]
- Kara M, Aydin T, Aran T, Turktekin N, Ozdemir B. Is dehydroepiandrosterone (DHEA) supplementation really effective on IVF‐ICSI outcome in patients with poor ovarian reserve?. Fertility and Sterility 2013;100(3, Supplement):S518. [Google Scholar]
Kim 2010 {published and unpublished data}
- Kim CH, Ahn JW, Nah HY, Kim SH, Chae HD, Kang BM. Ovarian features after 2 weeks, 3 weeks and 4 weeks transdermal testosterone gel treatment and their associated effect on IVF/ICSI outcome in low responders. Fertility and Sterility 2010;94(4 Suppl):S155‐6. [Google Scholar]
Kim 2011 {published data only}
- Kim CH, Howles CM, Lee HA. The effect of transdermal testosterone gel pretreatment on controlled ovarian stimulation and IVF outcome in low responders. Fertility and Sterility 2011;95(2):679‐83. [DOI] [PubMed] [Google Scholar]
Marzal 2014 {unpublished data only}
- Díaz C, Pellicer A, Marzal A. Antral Follicle Priming Prior to ICSI (Intracytoplasmic Sperm Injection) in Previously Diagnosed Low Responders (FOLLPRIM). https://clinicaltrials.gov/ct2/show/NCT01310647 (accessed 21 May 2014). [NCT01310647]
- Díaz C, Pellicer A, Marzal A. Antral Follicle Priming Prior to ICSI (Intracytoplasmic Sperm Injection) in Previously Diagnosed Low Responders (FOLLPRIM). personal correspondence 2014.
- Marzal A, Monterde M, Díaz‐García C, Gómez R, Rubio JM. Antral follicle priming prior to ICSI in confirmed low responders: a randomized clinical trial (FOLLPRIM). Human Reproduction 2014;29(Supp 1):i25. [Google Scholar]
Massin 2006 {published data only}
- Massin N, Cedrin‐Durnerin I, Coussieu C, Galey‐Fontaine J, Wolf JP, Hugues JN. Effects of transdermal testosterone application on the ovarian response to FSH in poor responders undergoing assisted reproduction technique‐‐a prospective, randomized, double‐blind study. Human Reproduction 2006;21(5):1204‐11. [DOI] [PubMed] [Google Scholar]
Moawad 2012 {published data only}
- Moawad M, Shaeer M. Long‐term androgen priming by use of dehydroepiandrosterone (DHEA) improves IVF outcome in poor‐responder patients. A randomized controlled study. Middle East Fertility Society Journal 2012;17(4):268–74. [Google Scholar]
Tartagni 2015a {published data only}
- Tartagni M, Pergola G, Damiani GR, Pellegrino A, Baldini D, Tartagni MV, et al. Potential benefit of dehydroepiandrosterone supplementation for infertile but not poor responder patients in a IVF program. Minerva Ginecologica 2015;67(1):7‐12. [PubMed] [Google Scholar]
Tartagni 2015b {published data only}
- Tartagni M, Cicinelli MV, Baldini D, Tartagni MV, Alrasheed H, DeSalvia MA, et al. Dehydroepiandrosterone decreases the age‐related decline of the in vitro fertilization outcome in women younger than 40 years old. Reproductive Biology and Endocrinology 2015;13:13‐8. [DOI: 10.1186/s12958-015-0014-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiser 2010 {published data only}
- Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor‐responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Human Reproduction 2010;25(10):2496‐500. [DOI] [PubMed] [Google Scholar]
Yeung 2013a {published data only}
- Yeung TWY, Li RHW, Lee VCY, Chai J, Ng EHY, Ho PC. A randomized double‐blinded placebo‐controlled trial on the effect of 16 weeks of dehydroepiandrosterone (DHEA) on ovarian reserve markers and IVF outcomes in normal responders. Fertility and Sterility 2013;100(3, Supplement):S114. [Google Scholar]
Yeung 2014 {published data only}
- Yeung TWY, Chai J, Li RHW, Lee VCY, Ho PC, Ng EHY. A randomized double‐blinded placebo‐controlled trial on the effect of 16 weeks of dehydroepiandrosterone (DHEA) on ovarian reserve markers and IVF outcomes in poor responders. Fertility and Sterility 2013;100(3, Suppl):S159‐60. [Google Scholar]
- Yeung TWY, Chai J, Li RHW, Lee VCY, Ho PC, Ng EHY. A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders. Fertility and Sterility 2014;102(1):108‐15.e1. [DOI: 10.1016/j.fertnstert.2014.03.044] [DOI] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang HH, Xu PY, Wu J, Zou WW, Xu XM, Cao XY, et al. Dehydroepiandrosterone improves follicular fluid bone morphogenetic protein‐15 and accumulated embryo score of infertility patients with diminished ovarian reserve undergoing in vitro fertilization: a randomized controlled trial. Journal of Ovarian Research 2014;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Balasch 2006 {published data only}
- Balasch J, Fábregues F, Peñarrubia J, Carmona F, Casamitjana R, Creus M, et al. Pretreatment with transdermal testosterone may improve ovarian response to gonadotrophins in poor‐responder IVF patients with normal basal concentrations of FSH. Human Reproduction 2006;21(7):1884‐93. [DOI] [PubMed] [Google Scholar]
Barad 2006 {published data only}
- Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Human Reproduction 2006;21(11):2845‐9. [DOI] [PubMed] [Google Scholar]
Barad 2007 {published data only}
- Barad D, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. Journal of Assisted Reproduction and Genetics 2007;24(12):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barad 2008b {unpublished data only}
- Barad D. A Trial of Dehydroepiandrosterone (DHEA) Treatment for in Vitro Fertilization (IVF). https://clinicaltrials.gov/ct2/show/NCT00419913 (accessed 12 March 2015). [NCT00419913]
Casson 2000 {published data only}
- Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Human Reproduction 2000;15(10):2129‐32. [DOI] [PubMed] [Google Scholar]
de los Santos 2013 {published data only}
- Santos MJ, García‐Laez V, Beltrán D, Labarta E, Zuzuarregui JL, Alamá P, et al. The follicular hormonal profile in low‐responder patients undergoing unstimulated cycles: is it hypoandrogenic?. Human Reproduction 2013;28(1):224‐9. [DOI] [PubMed] [Google Scholar]
Fábregues 2011 {unpublished data only}
- Fábregues F. Efficacy of Luteinizing Hormone (LH) Activity in Low Responder Patients With Transdermal Testosterone. https://clinicaltrials.gov/ct2/show/NCT01291212 (accessed 12 March 2015). [NCT01291212]
Fusi 2013 {published data only}
- Fusi FM, Ferrario M, Bosisio C, Arnoldi M, Zanga L. DHEA supplementation positively affects spontaneous pregnancies in women with diminished ovarian function. Gynecological Endocrinology 2013;29(10):940–3. [DOI] [PubMed] [Google Scholar]
Gleicher 2013 {published data only}
- Gleicher N, Kim A, Weghofer A, Shohat‐Ta A, Lazzaroni E, Lee HJ, et al. Starting and resulting testosterone levels after androgen supplementation determine at all ages in vitro fertilization (IVF) pregnancy rates in women with diminished ovarian reserve (DOR). Journal of Assisted Reproduction and Genetics 2013;30(1):49‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyman 2013 {published data only}
- Hyman JH, Margalioth EJ, Rabinowitz R, Tsafrir A, Algur N, Eldar‐Geva T. Dehydroepiandrosterone (DHEA) supplementation for poor responders ‐ how does it work?. Fertility and Sterility 2010;94(4, Supplement):S86. [Google Scholar]
- Hyman JH, Margalioth EJ, Rabinowitz R, Tsafrir A, Gal M, Alerhand S, et al. DHEA supplementation may improve IVF outcome in poor responders: a proposed mechanism. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;168(1):49–53. [DOI] [PubMed] [Google Scholar]
Monterde 2013 {published data only}
- Monterde M, Gomez R, Marzal A, Vega O, Rubio J, Diaz‐Garcia C, et al. Molecular expression of androgen and FSH receptors after follicular priming prior to ICSI cycles ‐ an interim analysis of the IFILL‐PRIMI randomised controlled trial. Human Reproduction 2013;28(Suppl 1):i342. [Google Scholar]
Motta 2006 {published data only}
- Motta EL, Rossi LM, Fernandes TR, Massaguer AA, Fassolas G, Serafini P. The use of DHEA in poor responders does not improve IVF outcomes: insights of a pilot study. Fertility and Sterility 2006;86(Suppl 2):S428. [Google Scholar]
Singh 2013 {published data only}
- Singh N, Zangmo R, Kumar S, Roy KK, Sharma JB, Malhotra N, et al. A prospective study on role of dehydroepiandrosterone (DHEA) on improving the ovarian reserve markers in infertile patients with poor ovarian reserve. Gynecological Endocrinology 2013;29(11):989–92. [DOI] [PubMed] [Google Scholar]
Sipe 2010 {published data only}
- Sipe CS, Thomas MR, Stegmann BJ, Voorhis BJ. Effects of exogenous testosterone supplementation in gonadotrophin stimulated cycles. Human Reproduction 2010;25(3):690‐6. [DOI] [PubMed] [Google Scholar]
Sönmezer 2009 {published data only}
- Sönmezer M, Ozmen B, Çil AP, Ozkavukçu S, Taşçi T, Olmuş H, et al. Dehydroepiandrosterone supplementation improves ovarian response and cycle outcome in poor responders. Reproductive BioMedicine Online 2009;19(4):508‐13. [DOI] [PubMed] [Google Scholar]
Yeung 2013b {published data only}
- Yeung TW, Li RH, Lee VC, Ho PC, Ng EH. A randomized double‐blinded placebo‐controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. Journal of Clinical Endocrinology and Metabolism 2013;98(1):380–8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Barad 2008a {unpublished data only}
- Study of Oral Dehydroepiandrosterone (DHEA) to Treat Previously Unexplained Infertility. Ongoing study March 2008.
Barad 2012 {unpublished data only}
- A Randomized Double Blind Control Trial of Transdermal Testosterone Supplementation vs Placebo on Follicular Development and Atresia, Oocyte and Embryo Quality Among Women With Diminished Ovarian Reserve Undergoing in Vitro Fertilization. Ongoing study July 2012End date: December 2014.
Jayaprakasan 2012 {published data only}
- Jayaprakasan K, Narkwichean A, Maalouf WE, Campbell BK. Efficacy of dehydroepiandrosterone to overcome the effect of ovarian ageing (DITTO): A proof of principle randomised controlled trial protocol. BMJ Open 2014;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolibianakis 2014 {unpublished data only}
- Transdermal Testosterone Pretreatment in Poor Responders Undergoing IVF. Ongoing study October 2013Estimated primary completion date: October 2014 (final data collection date for primary outcome measure).
Tsafrir 2007 {unpublished data only}
- DHEA Supplementation for Low Ovarian Response IVF Patients. Ongoing study Jan 2008.
Viardot‐Foucault 2012 {unpublished data only}
- Study of Dehydroepiandrosterone Treatment for Poor Responders in In Vitro Fertilization Patients. Ongoing study February 2012.
Vlahos 2014 {unpublished data only}
- Prospective Randomized Trial on the Effect of DHEA Administration in Women With Poor Ovarian Reserve Undergoing Controlled Ovarian Stimulation for IVF. Impact on Stimulation Characteristics and Pregnancy Outcome.. Ongoing study April 2014.
Additional references
Akhtar 2013
- Akhtar MA, Sur S, Raine‐Fenning N, Jayaprakasan K, Thornton JG, Quenby S. Heparin for assisted reproduction. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD009452.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosdou 2012
- Bosdou JK, Venetis CA, Kolibianakis EM, Toulis KA, Goulis DG, Zepiridis l, et al. The use of androgens or androgen‐modulating agents in poor responders undergoing in vitro fertilization: a systematic review and meta‐analysis. Human Reproduction Update 2012;18(2):127‐45. [DOI] [PubMed] [Google Scholar]
Buvat 2003
- Buvat J. Androgen therapy with dehydroepiandrosterone. World Journal of Urology 2003;21(5):346‐55. [DOI] [PubMed] [Google Scholar]
Casson 1998
- Casson PR, Santoro N, Elkind‐Hirsch K, Carson SA, Hornsby PJ, Abraham G, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin‐like growth factor‐I and decreases high‐density lipoprotein: a six‐month trial. Fertility and Sterility 1998;70(1):107‐10. [DOI] [PubMed] [Google Scholar]
Cheong 2013
- Cheong YC, Dix S, Hung Yu Ng E, Ledger WL, Farquhar C. Acupuncture and assisted reproductive technology. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD006920.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology 2010;63(8):834‐40. [DOI] [PubMed] [Google Scholar]
Davison 2005
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. Journal of Clincal Endocrinology and Metabolism 2005;90(7):3847‐53. [DOI] [PubMed] [Google Scholar]
Fouany 2013
- Fouany MR, Sharara FI. Is there a role for DHEA supplementation in women with diminished ovarian reserve?. Journal of Assisted Reproduction and Genetics 2013;30(9):1239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frattarelli 2004
- Frattarelli JL, Peterson EH. Effect of androgen levels on in vitro fertilization cycles. Fertility and Sterility 2004;81(6):1713‐4. [DOI] [PubMed] [Google Scholar]
Garcia‐Velasco 2005
- Garcia‐Velasco JA, Moreno L, Pacheco A, Guillén A, Duque L, Requena A, et al. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertility and Sterility 2005;84(1):82‐7. [DOI] [PubMed] [Google Scholar]
Genazzani 2001
- Genazzani AD, Stomati M, Strucchi C, Puccetti S, Luisi S, Genazzani AR. Oral dehydroepiandrosterone supplementation modulates spontaneous and growth hormone‐releasing hormone‐induced growth hormone and insulin‐like growth factor‐1 secretion in early and late postmenopausal women. Fertility and Sterility 2001;76(2):241‐8. [DOI] [PubMed] [Google Scholar]
Gleicher 2009
- Gleicher N, Ryan E, Weghofer A, Blanco‐Mejia S, Barad DH. Miscarriage rates after dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve: a case control study. Reproductive Biology and Endocrinology 2009;7:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gleicher 2011a
- Gleicher N, Barad D. Dehydroepiandrosterone. In: Kovacs G editor(s). How to improve your ART success rates. Cambridge: Cambridge University Press, 2011:93‐8. [Google Scholar]
Gleicher 2011b
- Gleicher N, Weghofer A, Barad DH. The role of androgens in follicle maturation and ovulation induction: friend or foe of infertility treatment?. Reproductive Biology and Endocrinology 2011;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gnoth 2005
- Gnoth C, Godehardt E, Frank‐Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Human Reproduction 2005;20(5):1144‐7. [DOI] [PubMed] [Google Scholar]
González‐Comadran 2012
- González‐Comadran M, Durán M, Solà I, Fábregues F, Carreras R, Checa MA. Effects of transdermal testosterone in poor responders undergoing IVF: systematic review and meta‐analysis. Reproductive BioMedicine Online 2012;25(5):450–9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horie 1992
- Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Human Reproduction 1992;7(10):1461‐6. [DOI] [PubMed] [Google Scholar]
Karp 2009
- Karp G, Bentov Y, Masalha R, Ifergane G. Onset of late posttraumatic seizure after dehydroepiandrosterone treatment. Fertility and Sterility 2009;91(3):931.e1‐2. [DOI] [PubMed] [Google Scholar]
Mamas 2009a
- Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertility and Sterility 2009;91(2):644‐6. [DOI] [PubMed] [Google Scholar]
Mamas 2009b
- Mamas L, Mamas E. Dehydroepiandrosterone supplementation in assisted reproduction: rationale and results. Current Opinion in Obstetrics and Gynaecology 2009;21(4):306‐8. [DOI] [PubMed] [Google Scholar]
Morales 1994
- Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. Journal of Clinical Endocrinology and Metabolism 1994;78(6):1360‐7. [DOI] [PubMed] [Google Scholar]
Nielsen 2011
- Nielsen ME, Rasmussen IA, Kristensen SG, Christensen ST, Møllgård K, Wreford Andersen E, et al. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Molecular Human Reproduction 2011;17(1):63‐70. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryan 1968
- Ryan KJ, Petro Z, Kaiser J. Steroid formation by isolated and recombined ovarian granulosa and thecal cells. Journal of Clinical Endocrinology & Metabolism 1968;28(3):355‐8. [DOI] [PubMed] [Google Scholar]
Sen 2010
- Sen A, Hammes SR. Granulosa cell‐specific androgen receptors are critical regulators of ovarian development and function. Molecular Endocrinology 2010;24(7):1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sir‐Petermann 2002
- Sir‐Petermann T, Maliqueo M, Angel B, Lara HE, Pérez‐Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Human Reproduction 2002;17(10):2573‐9. [DOI] [PubMed] [Google Scholar]
Siristatidis 2011
- Siristatidis CS, Dodd SR, Drakeley AJ. Aspirin for in vitro fertilisation. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD004832.pub3] [DOI] [PubMed] [Google Scholar]
Somboonporn 2005
- Somboonporn W, Davis SR, Seif MW, Bell RJ. Testosterone for peri‐ and postmenopausal women. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004509.pub2] [DOI] [PubMed] [Google Scholar]
Sunkara 2011
- Sunkara SK, Pundir J, Khalaf Y. Effect of androgen supplementation or modulation on ovarian stimulation outcome in poor responders: a meta‐analysis. Reproductive BioMedicine Online 2011;22(6):545‐55. [DOI] [PubMed] [Google Scholar]
Toner 2002
- Toner JP. Progress we can be proud of: U.S. trends in assisted reproduction over the first 20 years. Fertility and Sterility 2002;78(5):943‐50. [DOI] [PubMed] [Google Scholar]
Vendola 1999
- Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin‐like growth factor I expression and initiation of follicle development in the primate ovary. Biology of Reproduction 1999;61(2):353–7. [DOI] [PubMed] [Google Scholar]
Venetis 2011
- Venetis CA, Bosdou J, Kolibianakis E, Toulis K, Goulis D, Tarlatzis BC. Improved probability of pregnancy with transdermal testosterone pretreatment in poor responders treated with GnRH analogues and gonadotrophins for in‐vitro fertilization: a meta‐analysis. Fertility and Sterility 2011;96(3):S56. [Google Scholar]
Weil 1998
- Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, et al. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. Journal of Clinical Endocrinology and Metabolism 1998;83(7):2479‐85. [DOI] [PubMed] [Google Scholar]
Weil 1999
- Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle‐stimulating hormone interactions in primate ovarian follicle development. Journal of Clinical Endocrinology and Metabolism 1999;84(8):2951‐6. [DOI] [PubMed] [Google Scholar]
Yakin 2011
- Yakin K, Urman B. DHEA as a miracle drug in the treatment of poor responders; hype or hope?. Human Reproduction 2011;26(8):1941–4. [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2009
- Zegers‐Hochschild F, Adamson GD, Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertility and Sterility 2009;92(5):1520‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Nagels 2012
- Nagels HE, Rishworth JR, Siristatidis CS, Kroon B. Androgens (dehydroepiandrosterone or testosterone) in women undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009749] [DOI] [PMC free article] [PubMed] [Google Scholar]


